Research Article
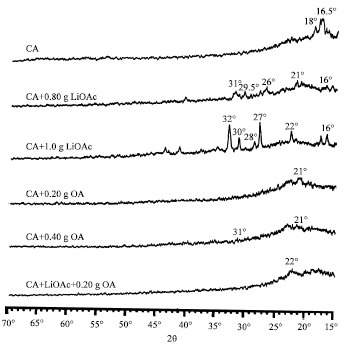
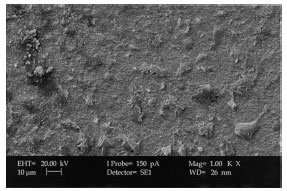
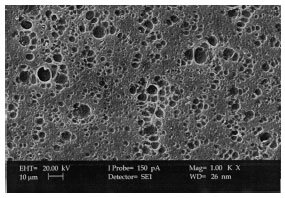
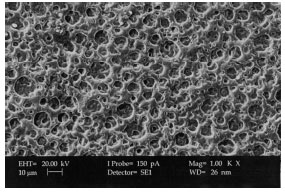
XRD and Surface Morphology Studies on Chitosan-Based Film Electrolytes
Faculty of Applied Sciences, Universiti Teknologi MARA, 40450 Shah Alam, Malaysia
M.K. Harun
Faculty of Applied Sciences, Universiti Teknologi MARA, 40450 Shah Alam, Malaysia
A.M.M. Ali
Faculty of Applied Sciences, Universiti Teknologi MARA, 40450 Shah Alam, Malaysia
M.F. Mohammat
Faculty of Applied Sciences, Universiti Teknologi MARA, 40450 Shah Alam, Malaysia
M.A.K.M. Hanafiah
Faculty of Applied Sciences, Universiti Teknologi MARA, 40450 Shah Alam, Malaysia
S.C. Ibrahim
Faculty of Applied Sciences, Universiti Teknologi MARA, 40450 Shah Alam, Malaysia
M. Mustaffa
Faculty of Applied Sciences, Universiti Teknologi MARA, 40450 Shah Alam, Malaysia
Z.M. Darus
Faculty of Applied Sciences, Universiti Teknologi MARA, 40450 Shah Alam, Malaysia
F. Latif
Faculty of Applied Sciences, Universiti Teknologi MARA, 40450 Shah Alam, Malaysia