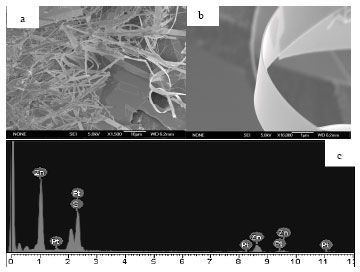
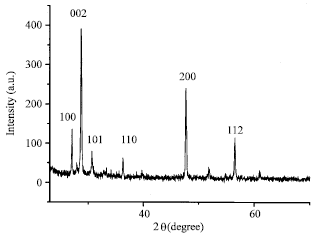
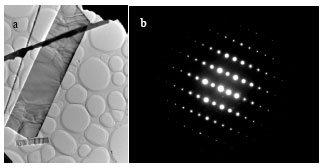
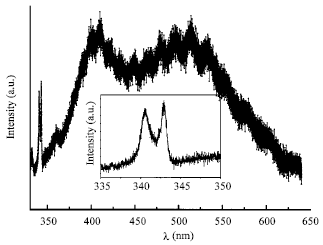
Research Article
Synthesis of ZnS Nanobelts with Multi Photoluminescence Peaks
Department of Physics, Central China Normal University, Wuhan 430079, Peoples Republic of China
Xintang Huang
Department of Physics, Central China Normal University, Wuhan 430079, Peoples Republic of China
Ming Tang
Department of Physics, Central China Normal University, Wuhan 430079, Peoples Republic of China