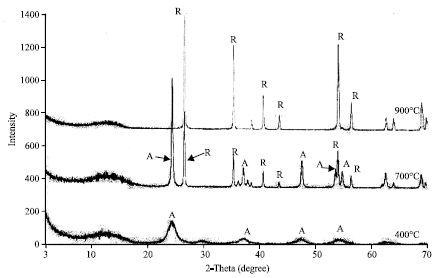
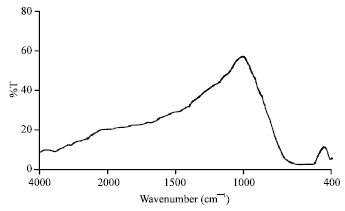
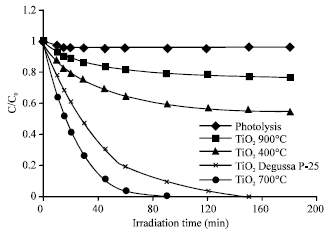
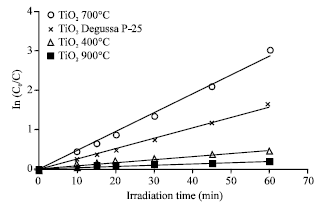
Research Article
Preparation of TiO2 Photocatalyst Using TiCl4 as a Precursor and its Photocatalytic Performance
Laboratory of Chemistry-Physics, Department of Chemistry, Faculty of Sciences, University Ibn Zohr, B.P. 8106 Hay Dakhla, Agadir, Morocco
M. Tamimi
Laboratory of Chemistry-Physics, Department of Chemistry, Faculty of Sciences, University Ibn Zohr, B.P. 8106 Hay Dakhla, Agadir, Morocco
A. Assabbane
Laboratory of Chemistry-Physics, Department of Chemistry, Faculty of Sciences, University Ibn Zohr, B.P. 8106 Hay Dakhla, Agadir, Morocco
A. Bouamrane
Environmental Analysis Laboratory of the Processes and the Industrial Systems, Institutes National Sciences Applied of Lyon, 7 Street of Physics, 69621 Villeurbanne Cedex, France
A. Nounah
Laboratory of Environment Sciences, Technology High School, B P. 227, Sale-Medina, Morocco
L. Laanab
Centre of Microscopy, Faculty of Sciences, University Mohammed V, B.P. I C) 14 Agdal, Rabat, Morocco
Y. Ait-Ichou
Laboratory of Chemistry-Physics, Department of Chemistry, Faculty of Sciences, University Ibn Zohr, B.P. 8106 Hay Dakhla, Agadir, Morocco