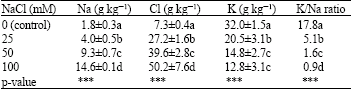
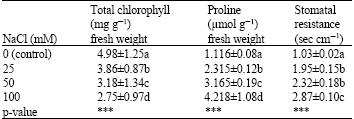
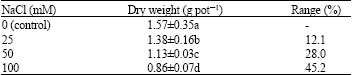Research Article
Effect of NaCl on Stomatal Resistance and Proline, Chlorophyll, Na, Cl and K Concentrations of Lentil Plants
Department of Soil Science, Faculty of Agriculture, Uludag University, 16059 Bursa, Turkey
Nilufer Turkmen
Department of Soil Science, Faculty of Agriculture, Ankara University, (361 1 C) Ankara, Turkey
Nilgun Taban
Department of Soil Science, Faculty of Agriculture, Ankara University, (361 1 C) Ankara, Turkey