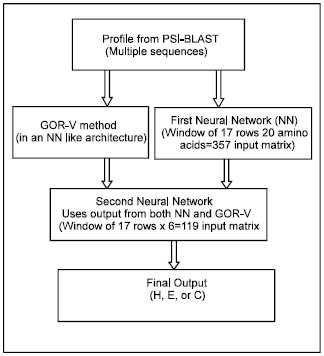
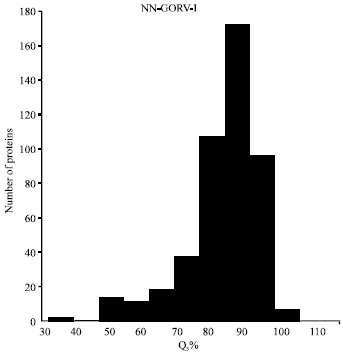
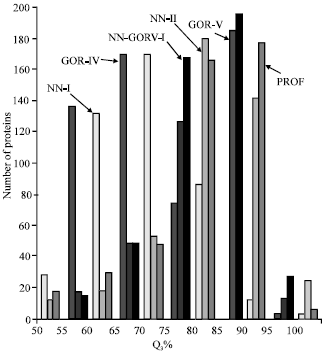
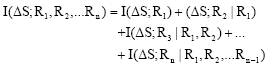Research Article
A Hybrid Classifier for Protein Secondary Structure Prediction
College of Computing, Al Ghurair University, Dubai, United Arab Emirates
Safaai Deris
School of Graduates Studies, University Teknologi Malaysia, UTM Skudai, Johor, Malaysia
Mohd Saberi Mohamad
School of Graduates Studies, University Teknologi Malaysia, UTM Skudai, Johor, Malaysia