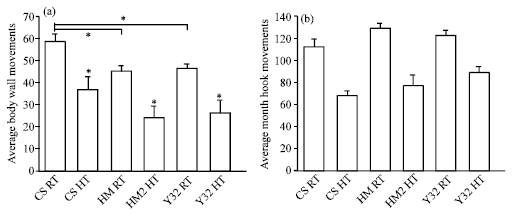
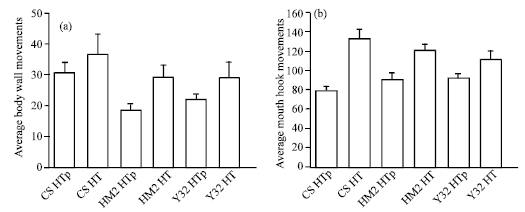
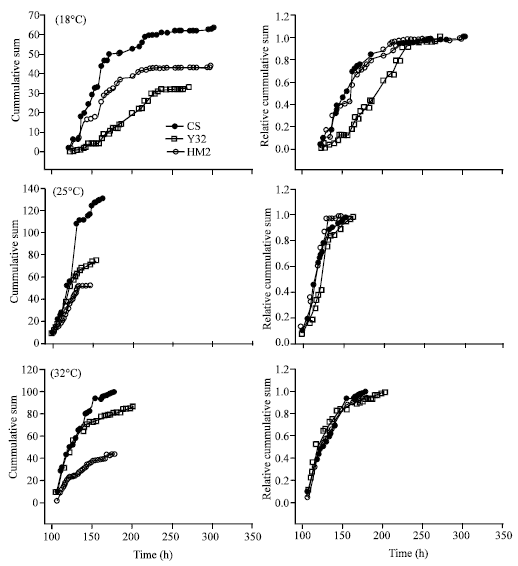
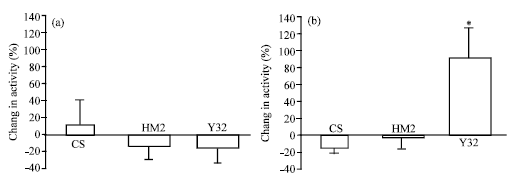
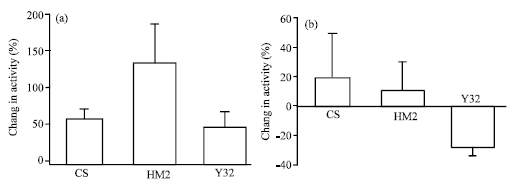
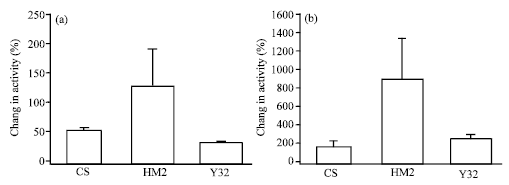
Research Article
Reduced and Misexpression of 5-HT2 Receptors Alters Development, Behavior and CNS Activity in Drosophila melanogaster
Department of Biology, University of Kentucky, Lexington, KY 40506-0225, United States of America
L. Wang
Department of Biology, University of Kentucky, Lexington, KY 40506-0225, United States of America
D.A. Harrison
Department of Biology, University of Kentucky, Lexington, KY 40506-0225, United States of America
R.L. Cooper
Department of Biology, University of Kentucky, Lexington, KY 40506-0225, United States of America