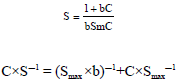Research Article
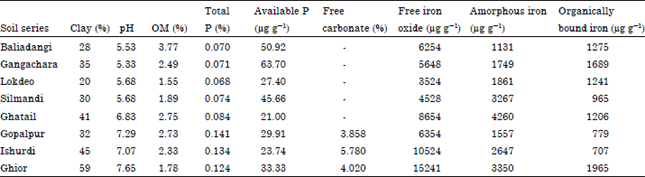
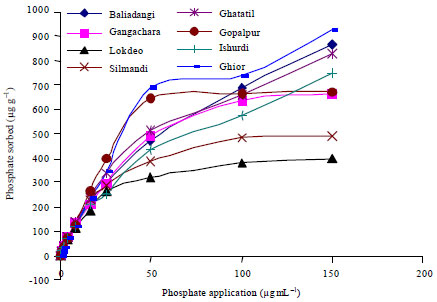
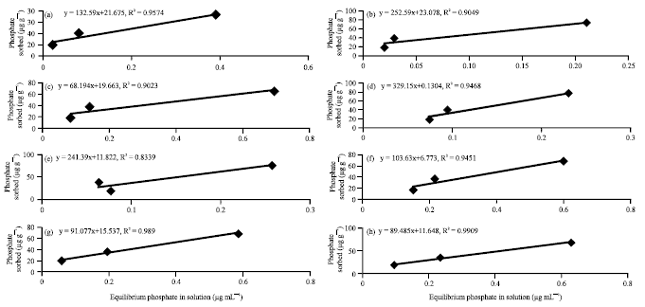
A Comparison of the Langmuir, Freundlich and Temkin Equations to Describe Phosphate Sorption Characteristics of Some Representative Soils of Bangladesh
Department of Soil Science, University of Chittagong, Bangladesh
Sirajul Hoque
Department of Soil, Water and Environment, University of Dhaka, Bangladesh
K.T. Osman
Department of Soil Science, University of Chittagong, Bangladesh