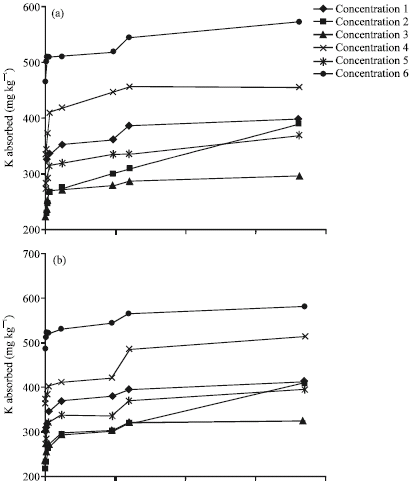
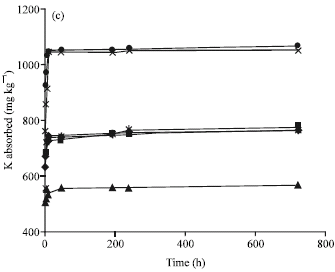
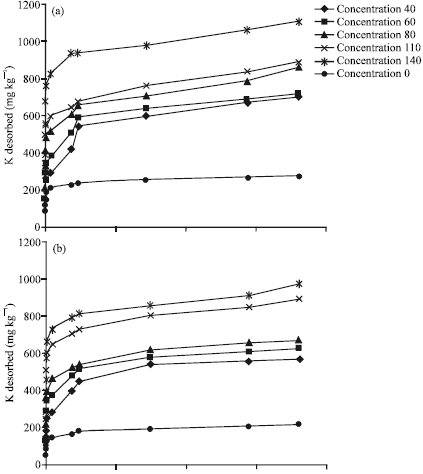
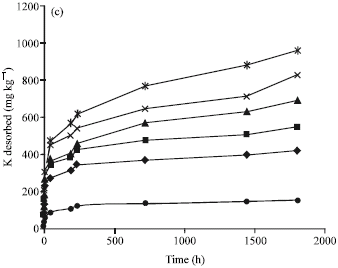
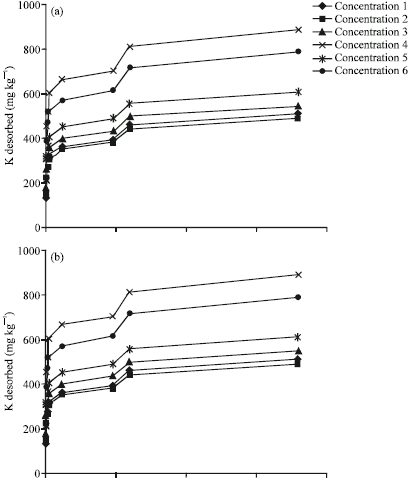
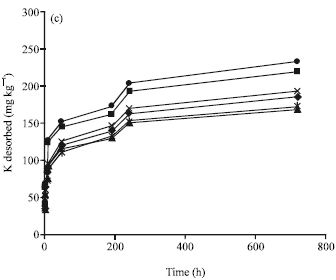
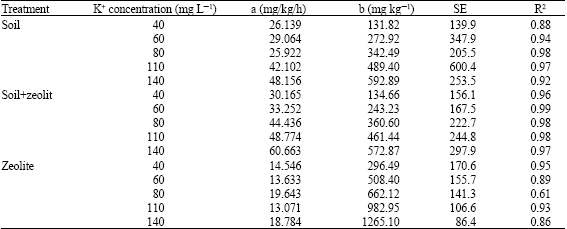
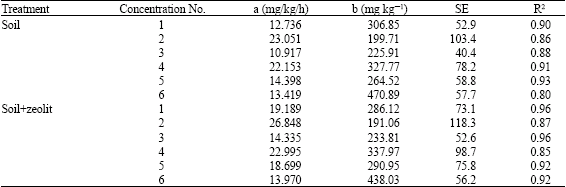
Research Article
Effects of Ammonium and Iranian Natural Zeolite on Potassium Adsorption and Desorption Kinetics in the Loess Soil
Department of Soil Science, Gorgan University of Agricultural Sciences and Natural Resources, Golestan Province, Iran
S.A.R. Movahedi Naeini
Department of Soil Science, Gorgan University of Agricultural Sciences and Natural Resources, Golestan Province, Iran