Research Article
Effect of Sodium Azide on the Growth and Variability Induction in Helianthus annuus L.
Department of Horticulture (Ornamental Plants), Faculty of Agriculture, South Valley University, Qena, Egypt
There are about 70 species of Helianthus, some of them are perennials and many are popular garden ornamentals. Helianthus tuberosus grows throughout the world for its edible tubers; beach sunflower (H. debilis) is used as a border plant and ground cover. Helianthus annuus is one of the most important species belongs to the family Asteraceae (compositae), popularly known as the sunflower. All sunflowers are good and long lasting as cut flowers. Sunflowers are widely grown commercially for the oil that is extracted from the seeds which contain 35-40% oil. They are high in polyunsaturated fat and contain no cholesterol. Seeds also are used to feed the birds (Gvozdenovic et al., 2009).
Induced mutation using physical and chemical mutagen is an method to create genetic variation resulting in new varieties with better characteristics (Wongpiyasatid et al., 2000; Arulbalachandran et al., 2009).
Sodium azide (NaN3) is a chemical mutagen and which considers as one of the most powerful mutagens in plants. Its application on plant is easy and inexpensive and creates mutation to improve their traits. The efficiency of mutant production depends on many conditions such as pH, soaking into water, temperature, concentration of azide and treatment duration. It creates point mutation and damages the chromosomes and thus produces tolerance in the plants for numerous adverse conditions (Al-Qurainy and Khan, 2009).
Sodium azide was used in many studies to induce mutation as found by Akhaury et al. (1996) on Hordium vulgare; Bhate (1999) on Ipomea purpurea; El-Nashar (2006) on Amaranthus caudatus and Al-Gawwad and Makka (2009) on Mirabilis jalapa.
Many searches were made to propagate sunflower by tissue culture technique. Hosoki et al. (1994) stated that nodes could be rooted in MS medium with 0.1 mg Indole-3-Butyric Acid (IBA)/liter. While, Paterson (1984) reported that shoot multiplication of Helianthus annuus was optimal from half shoot apices cultured on MS media with 0.1-1.0 mg L-1 benzyl adenine or kinetin. Auxins inhibited multiplication and promoted callusing. Rooting was poor and was not promoted by auxins.
The aim of this research was to study the effect of sodium azide on the growth of sunflower. Also, to produce genetic variation in the vegetative and flowering growth as a new pattern.
The study was carried out at the Nursery of Ornamental Plants, Faculty of Agriculture, South Valley University, Qena, Egypt during 2008, 2009 and 2010.
Two cultivars of Helianthus annuus Giza 1 and 102 were used in this study. Seeds were obtained from the Agriculture Research Station, Qena. At first, seeds of sunflower were soaked in sodium azide concentrations of 0, 200, 400, 600, 800 and 1000 ppm for 24 h. No seed germination was observed. The seeds were then soaked in sodium azide solutions with the concentrations of 0, 100, 200, 300, 400 and 500 ppm for 4 h. The pH of solutions was adjustified at 3.6 using orthophosphoric acid. One hundred and twenty seeds per each treatment were sown directly in the field. The seeds were sown in three replications, each replication contained four ridges, five holes in each one (two seeds in each hole) with distance of 30 cm between plants.
Seeds of first generations (M1) were sown for two seasons in 31 May, 2008 and 28 July, 2009 for first and second seasons, respectively. Seeds obtained from open pollinated for M1 plants were sown as M2 generation in 28 July, 2009 and 20 February, 2010 for first and second seasons, respectively.
All plants of the different treatments were examined daily to search for variation in the vegetative and flowering growth.
Statistical analysis: All obtained data were subjected to the analysis of variance according to the procedure outlined by Steel and Torrie (1982). The differences between the different treatments means were compared using the least significant differences (LSD) at 0.05%.
Recorded data: Seeds germination percentage as a mean was calculated after one week. This data were subjected to angular transformation prior to statistical analysis. At flowering stage: plant height, no of leaves, stem diameter, flowering date, no of ray florets/inflorescence, flower longevity, fresh weight and dry weight of vegetative growth were recorded. No. of seeds/head were also recorded at the maturity stage.
Valuable mutant obtained were cultured in the tissue culture laboratory in qena to propagate it. The plants were washed with tap water and then washed with liquid detergent for 15 min then plant was washed with 70% ethanol for 5 sec at the laminar air flow. After washing with autoclaved double distilled water, they were treated with 0.1% mercuric chloride for 10 min and washed again for six times with this water to remove any trace of mercuric chloride. Leaves and nodes are the two types of explants which were cultured on MS medium supplemented with 0.05 BA and 0.05 IBA with or without 1.0 mg L-1 AgNO3 for shoot induction. Ms medium supplemented with 0.1 IBA were used for rooting. The medium was autoclaved at 1.0 kg cm-2 for 20 min. Cultures were incubated at 10000 lux with cool white fluorescent light and maintained at 25°C.
At first, seeds of sunflower were soaked in sodium azide concentrations of 0, 200, 400, 600, 800 and 1000 ppm for 24 h. No seed germination was observed, this may be due that the higher dose of sodium azide cause disturbance in the genetically and physiological activities leading to the death of the cells as reported by Al-Qurainy and Khan (2009).
With using the concentrations of 0, 100, 200, 300, 400 and 500 ppm for 4 h, the results were as follows:
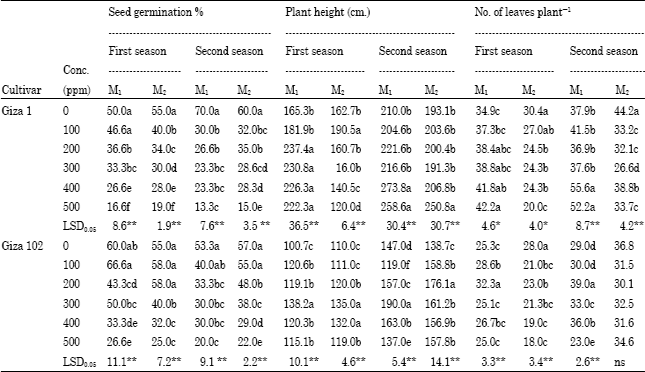
With respect to cv. Giza 1, seed germination percentages were decreased significantly using all sodium azide concentrations for both M1 and M2 generations in the two seasons as shown in Table 1.
For cv. Giza 102, seeds treated with 100 ppm sodium azide did not differ significantly from the control in germination percentage in both seasons for M1 and M2. Similar, result was obtained after 200 ppm treatment for M2 in the first season. Other treatments decreased significantly seed germination %.
These slightly decrease in seed germination percentage with high concentrations of sodium azide was supported by the results of Khan et al. (2004, 2005), Sinha and Lai (2007) and Al-Gawwad and Makka (2009). Reduction in seed germination as a result of mutagenic treatments has been explained due to delayed or inhibition in physiological and biological processes necessary for seed germination which include enzyme activity as reported by Al-Qurainy and Khan (2009).
The high concentrations of 200, 300, 400, 500 ppm sodium azid increased significantly M1 plant height in cv.Giza 1 in the first season compared to the control (237.4 and 165.3, respectively) as shown in Table 1. The highest plant height were obtained in plants treated with 100 ppm in the first season for M2, 400 ppm in the second season for M1 and 500 ppm for M2 in the second season compared to the control (190.5, 273.8 and 250.8, respectively).
| Table 1: | Effect of sodium azide on the seed germination %, plant height and No. of leaves plant-1 of Helianthus annuus |
 | |
| ns,* and **Not significant, significant at 0.05 and 0.01, respectively. Values in the same column not followed by the same letter are significantly different at the 5 % level of probability | |
Regarding to cv. Giza 102, the treatment of 300 ppm increased plant height for M1 and M2 in the first season and M1 in the second one (138.2, 135, 190, respectively) compared to the control. This result confirms with the result of El-Nashar (2006) that found the SA improved growth of the Amaranthus seedlings.
For cv. Giza 1, the treatments of 400 and 500 ppm sodium azide increased significantly the No of leaves in the M1 for both seasons compared to the control as shown in Table 1. These increments may be due to the physiological stimulation of the chemical mutagen as reported by El-Torky (1992). In the M2 for both seasons, all treatments decreased the No of leaves compared to the control.
Plants of cv. Giza 102 treated with 200 ppm gave the largest No of leaves in both seasons for M1. On the other hand, No of leaves was decreased using all concentrations of sodium azide in the M2 plants of the first season, while it did not differ significantly from the control in the second season. This result may be due to mutagenic damage which depended on the biological traits of the variety as reported by Gvozdenovic et al. (2009) on sunflower.
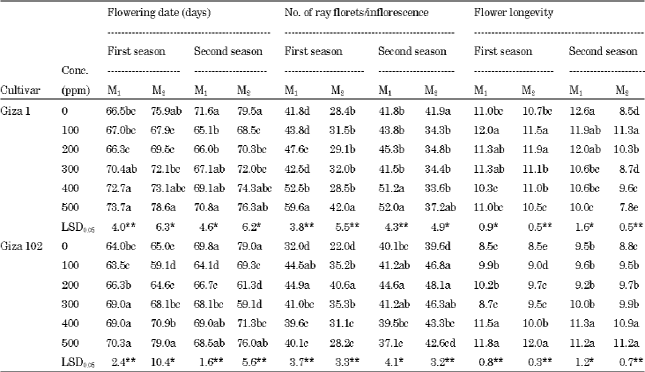
Plants of cv. Giza 1 treated with low concentrations of 100 and 200 ppm sodium azide flowered early as the control plants, while the high concentrations delayed flowering in both generations of both seasons as shown in Table 2. Similar results were found concerning to cv. Giza 102 plants. This result agrees with the results of El-Nashar (2006) and Khan et al. (2006).
The two cultivars of sunflower: cv. Giza 1 and 102 plants treated with 500 ppm sodium azide gave the highest number of ray florets/inflorescence in the two generations of both seasons as shown in Table 2. This result in the line with the finding of El-Nashar (2006).
The concentration of 200 ppm sodium azide increased significantly the number of ray florets/inflorescence of cv. Giza 102 in both generations of both seasons compared to the control. This result was in harmony with that reported by Al-Gawwad and Makka (2009).
| Table 2: | Effect of sodium azide on the flowering date, No. of ray florets/inflorescence and flower longevity of Helianthus annuus |
 | |
| *** and **Significant at 0.05 and 0.01, respectively. Values in the same column not followed by the same letter are significantly different at the 5 % level of probability | |
The data shown in Table 2 indicated that the two genotypes Giza 1 and 102 produced a wide range of response to sodium azide concentrations for flower longevity. With respect to Giza 1, the low concentrations increased flower longevity. Contrary, the high concentrations increased flower longevity in Giza 102 in both generations of the two seasons.
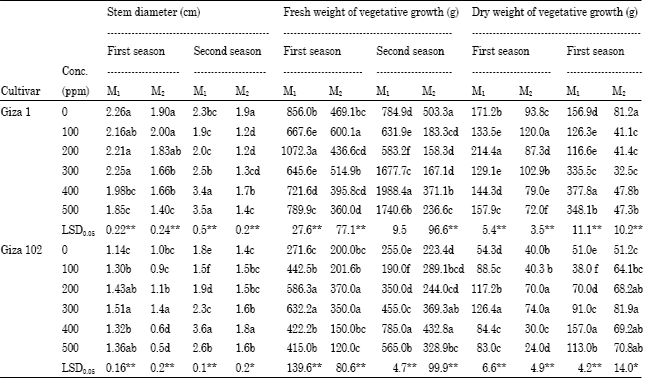
The concentrations of 400 and 500 ppm decreased stem diameter of cv. Giza 1 plants in both generations of the first season as shown in Table 3, while these concentrations increased the stem diameter in the M1 of the second season. All concentrations of sodium azide decreased stem diameter in the M2 of the second season compared with the control.
For cv. Giza 102 plants, the concentration of 300 ppm gave the largest stem diameter (1.51 and 1.4) compared to control (1.14 and 1.0, respectively). In the M1 and M2 for second season, the concentration of 400 ppm gave the highest stem diameter (3.6 and 1.8) compared to control (1.8 and 1.4, respectively).
The results of the fresh and dry weights were shown in Table 3. For Giza1, it can be shown that the concentration of 200 and 100 ppm gave the highest fresh and dry weights in the first season for M1 and M2 (1072.3 and 600.1 for fresh weight, 2.14 and 120 for dry weight, respectively) compared to the control (856 and 469.1 for fresh weight and 171.2 and 93.8 for dry weights, respectively). Seeds treated with 400 ppm gave the highest fresh and dry weight in the second season for M1, while in the M2, all treatments decreased fresh and dry weights.
Concerning to cv. Giza 102, the concentrations of 200 and 300 gave the highest fresh and dry weight in the first season for M1 and M2 generations, whereas the concentration of 400 ppm was the best for increasing fresh and dry weights in the second season for both generations compared to the control. These results confirmed with the results of El-Nashar (1998, 2006) and Al-Gawwad and Makka (2009).
| Table 3: | Effect of sodium azide on the stem diameter, fresh and dry weight of the vegetative growth of Helianthus annuus |
 | |
| ** and **Significant at 0.05 and 0.01, respectively. Values in the same column not followed by the same letter are significantly different at the 5 % level of probability | |
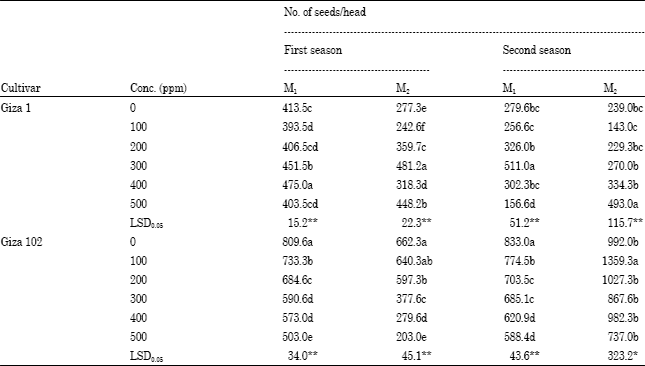
The number of seeds per head for cv. Giza 102 decreased significantly compared to the control with increasing the concentration of sodium azide in both generations of first season and for M1 in the second season as shown in Table 4. The concentration of 100 ppm increased the number of seeds in the M2 in the second season compared to the control.
With respect with cv. Giza 1 plants, the concentrations 400, 300, 300 500 gave the highest No. of seeds per head in the first season for M1 and M2 and during second season for both generations respectively.
In the M1 generation of first season, all the SA treatments produced changes in the leaf form in Giza 1 as shown in Fig. 1a and b. The concentration of 300 ppm produced plant with reddish leaf petiole in Giza 1 as shown in Fig. 2. Changes in the inflorescence form were found using 300 ppm in Giza 102 as shown in Fig. 3a and b. All this variations disappeared in the M2 obtained after self-pollination.
| Table 4: | Effect of sodium azide on the No. of seeds/head of Helianthus annuus |
 | |
| *Significant at 0.01. Values in the same column not followed by the same letter are significantly different at the 5 % level of probability | |
 | |
| Fig. 1: | (a, b) Photograph showing changes in the leaf form in the M1 of the first season as a result of the treatment with sodium azide (from left to right: control, 100, 100, 200, 300, 400 and 500, respectively |
 | |
| Fig. 2: | Photograph showing reddish leaf petiole in the M1 of the first season as a result of the treatment with sodium azide at 300 ppm |
 | |
| Fig. 3: | (a, b) Photograph showing changes in the inflorescence form in the M1 of the first season as a result of the treatment with sodium azide at 300 ppm |
These changes could be referred to the layer rearrangement as a result of the chemical mutagen effect as stated by Abd El-Maksoud (1988) and El-Nashar (2006).
Plant of Giza 1 having inflorescence calyx with green dark colour was obtained after treatment of 300 ppm in the M2 generation of the first season as shown in Fig. 4. Also, plant with stripped ray florets by white colour was also found using 200 ppm as shown in Fig. 5. The treatments of 100 and 200 ppm produced dwarfed plants in Giza 102. Also, dwarfed plants were produced using 200 and 400 ppm in Giza 1.
 | |
| Fig. 4: | Photograph showing abnormal inflorescence and its calyx having dark green colour in the M2 of the first season as a result of the treatment with sodium azide at 300 ppm |
 | |
| Fig. 5: | Photograph showing inflorescence with stripped ray florets in the M2 of first season as a result of the treatment with sodium azide at 200 ppm |
The treatment of 100 ppm sodium azide produced five plants with many inflorescence in Giza 102 as shown in Fig. 6a and b. The inflorescence number in the plant varied between 24 to 43 inflorescences per plant. Their flower diameter varied between 3.5 to 15.2 cm. The seeds number also varied between 280 to 950 seeds per head for each plant. This character transmitted to the progeny. So, it can be propagated as a new cultivar.
In the M2 generation of the second season, four variegated plants of cv. Giza 1 were observed in 100 and 200 ppm sodium azide treatments as shown in Fig. 7a-d. These variegated leaves could be attributed to one of the following reasons: (1) the epidermal layer lacked chlorophyll and the internal tissues also lack of chlorophyll because epidermal cells have displaced inner cells in particular regions, the result was apatchy green. (2) The variegation may be caused by gene and/or plant plastid changes as a result of the mutagen agent treatment. This result confirmed the finding of Guimaras and Ando (1981), who stated the greater mutagenic efficiency of sodium azide in producing chlorophyll mutations. One of these plants reached flowering but without producing any seeds. Two of them died before anthesis. One of them was used in the tissue culture laboratory to propagate it. Leaves and nodes were cultured on MS medium supplemented with 0.05 BA and 0.05 IBA with or without 1.0 mg L-1 Ag NO3. Shoots were obtained from node cultured in MS medium supplemented with equal concentration from BA and IBA without silver nitrate as shown in Fig. 8. These shoots failed to root in MS medium supplemented with 0.1 IBA.
 | |
| Fig. 6: | (a, b) Photograph showing plants with many inflorescences of cv. Giza 102 in the M2 of the first season as a result of the treatment with sodium azide at 100 ppm |
 | |
| Fig. 7: | (a-d) Photograph showing plants of cv. Giza 102 having variegated leaves obtained in the second season for M2 as a result of the treatment with sodium azide at 100 and 200 ppm |
 | |
| Fig. 8: | Photograph showing shoots raised from culture nod of variegated plant |
Sodium azide had a powerful mutagen effects on Helianthus annuus. It was created dwarfed and variegated plants. Also, plants with many inflorescences were obtained and this trait was inherited to next generation. Selection must be taken place to propagate it as new cultivars.