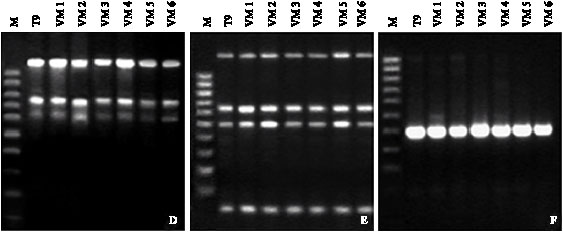
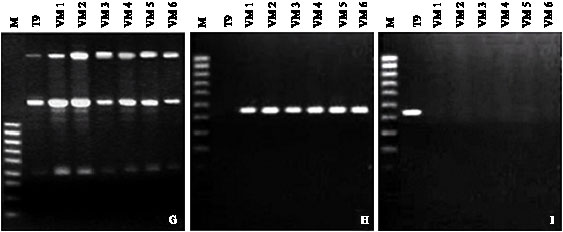
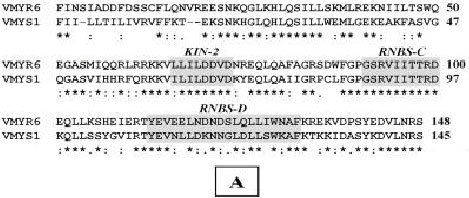
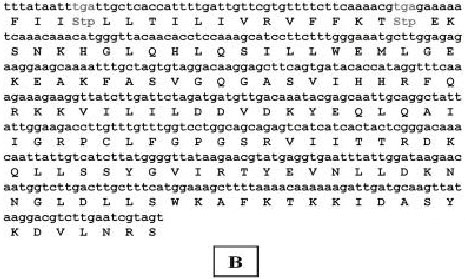
Research Article
Agronomic, Genetic and Molecular Characterization of MYMIV-Tolerant Mutant Lines of Vigna mungo
Plant Molecular and Cellular Genetics Section, Bose Institute, P 1/12 CIT Scheme VII-M, Kolkata 700054, India
J. Basak
Plant Molecular and Cellular Genetics Section, Bose Institute, P 1/12 CIT Scheme VII-M, Kolkata 700054, India
S. Maiti
Plant Molecular and Cellular Genetics Section, Bose Institute, P 1/12 CIT Scheme VII-M, Kolkata 700054, India
A. Kundu
Plant Molecular and Cellular Genetics Section, Bose Institute, P 1/12 CIT Scheme VII-M, Kolkata 700054, India
B. Das
Department of Botany, Bose Institute, 93/1 APC Road, Kolkata-700009, India
T.K. Ghose
Department of Botany, Bose Institute, 93/1 APC Road, Kolkata-700009, India
A. Pal
Plant Molecular and Cellular Genetics Section, Bose Institute, P 1/12 CIT Scheme VII-M, Kolkata 700054, India