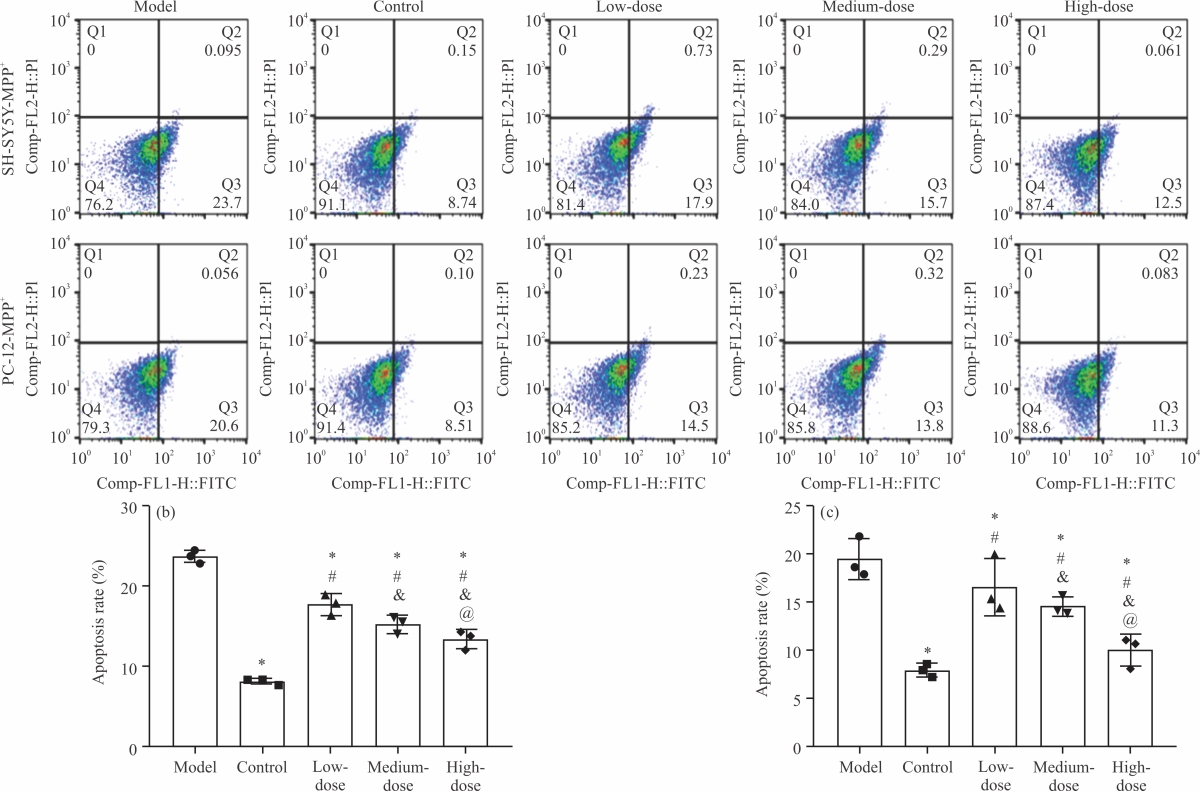
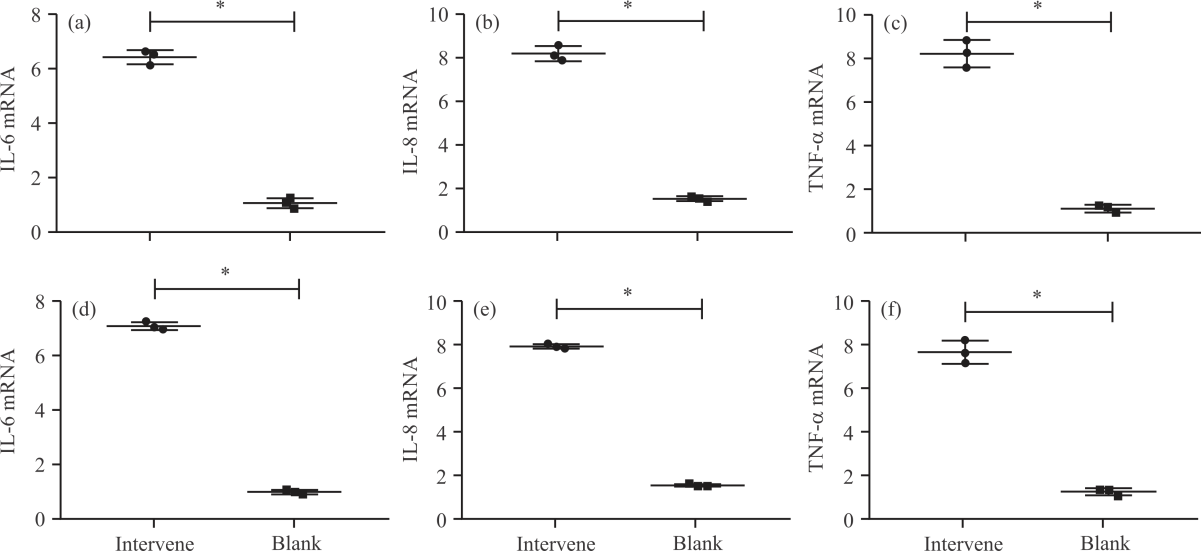
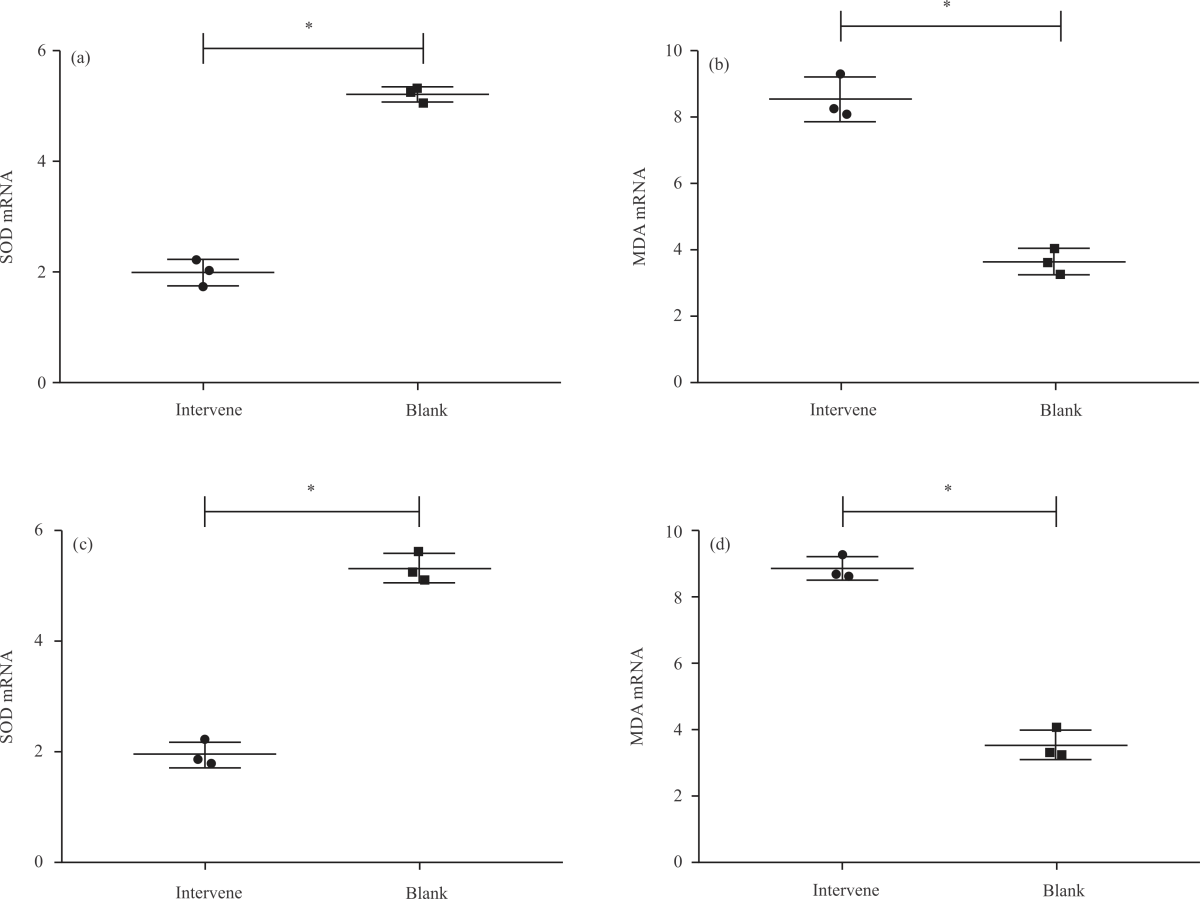
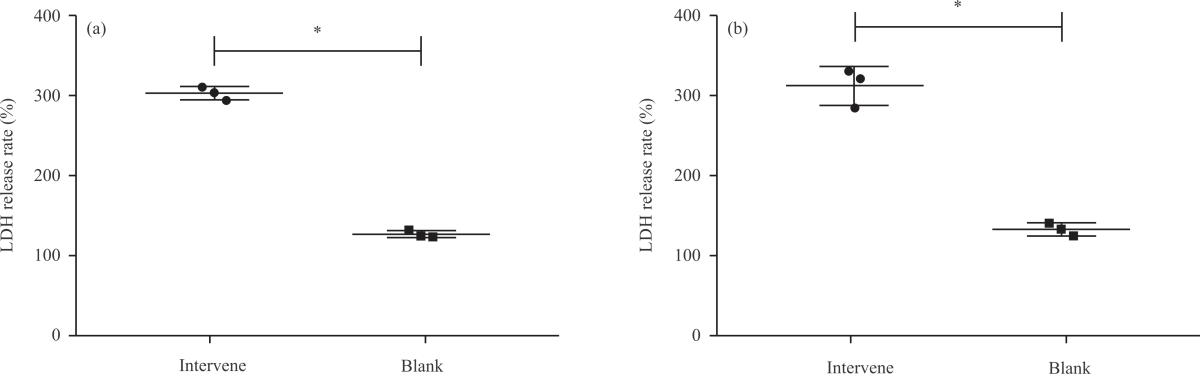
Research Article
Influence of Nervonic Acid on Parkinson’s Disease Model Cells through Ras/MEK/ERK Axis
School of Life Science, Northwest Normal University, Lanzhou, Gansu 730070, China
Yujuan Cui
School of Life Science, Northwest Normal University, Lanzhou, Gansu 730070, China
Ji Zhang
School of Life Science, Northwest Normal University, Lanzhou, Gansu 730070, China
LiveDNA: 86.37984