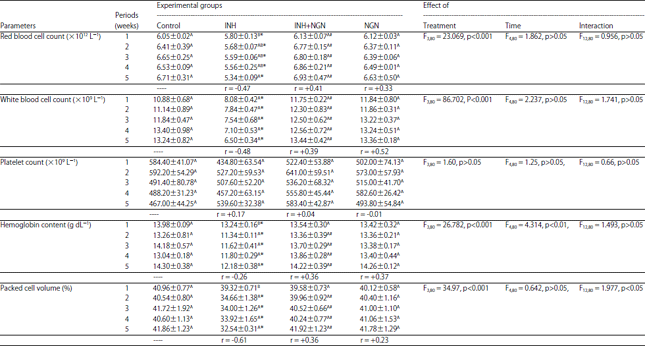
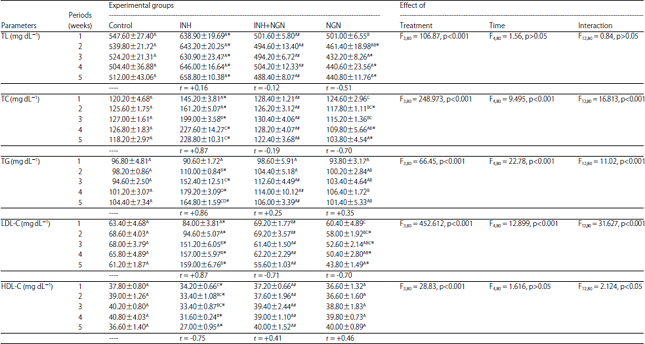
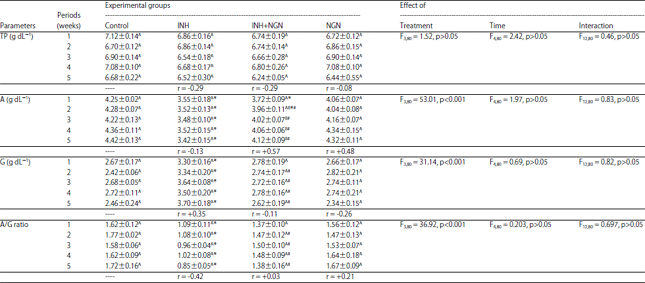
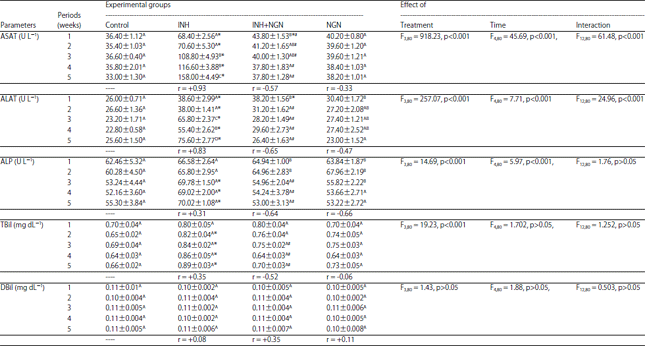
Research Article
Protective Effect of Naringenin Against Isoniazid-induced Adverse Reactions in Rats
Division of Physiology, Department of Zoology, Faculty of Science, Cairo University, Giza, Egypt
Atef Abdel-Moneem Ali
Division of Physiology, Department of Zoology, Faculty of Science, Cairo University, Giza, Egypt
LiveDNA: 20.20388
Sara Abdel-Mohsen Soliman
National Research Center (NRC), Dokki, Giza, Egypt