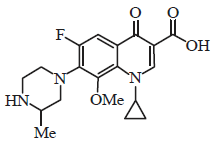
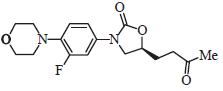
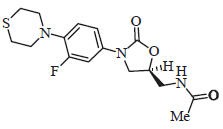
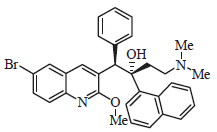
Review Article
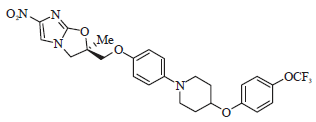
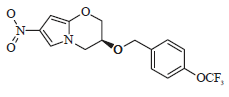
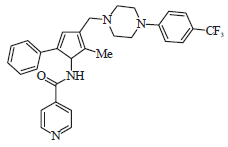
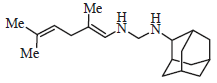
Advances in the Treatment Options Towards Drug-Resistant Tuberculosis
Department of Pharmacy, Clloege of Medicine and Health Sciences, Ambo University, Ambo, Ethiopia
Faheem Maqbool
Faculty of Pharmaceutical Sciences, Government College University, Faisalabad, Pakistan
Ishtiaq Ahmed
School of Medical Science, Gold Coast Campus, Griffith University, Southport QLD 4222, Australia
Kuldeep Dhama
Division of Pathology, ICAR-Indian Veterinary Research Institute, Izatnagar, 243122 Bareilly, Uttar Pradesh, India
LiveDNA: 91.4710
Hafiz Muhammad Nasir Iqbal
School of Engineering and Science, Tecnologico de Monterrey, Campus Monterrey, Ave. Eugenio Garza Sada 2501, Monterrey, N.L., CP 64849, Mexico