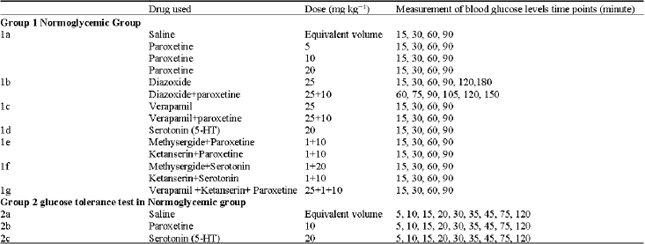
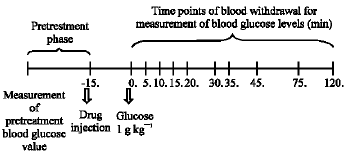
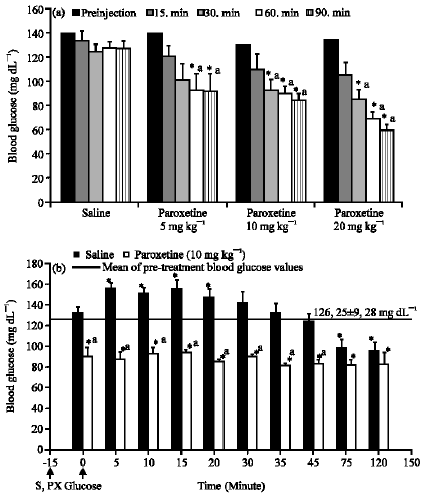
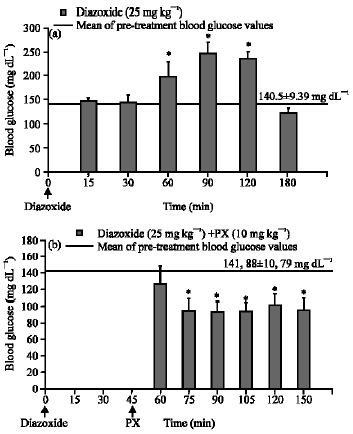
Research Article
The Effect of Paroxetine, A Selective Serotonin Reuptake Inhibitor, on Blood Glucose Levels in Mice
Department of Pharmacology, Faculty of Medicine, Karadeniz Technical University, Mine Kadioglu, TR-61187 Trabzon, Turkey
E. Muci
Department of Pharmacology, Faculty of Medicine, Karadeniz Technical University, Mine Kadioglu, TR-61187 Trabzon, Turkey
M. Kesim
Department of Pharmacology, Faculty of Medicine, Karadeniz Technical University, Mine Kadioglu, TR-61187 Trabzon, Turkey
C. Ulku
Department of Pharmacology, Faculty of Medicine, Karadeniz Technical University, Mine Kadioglu, TR-61187 Trabzon, Turkey
E. N. Duman
Department of Anesthesiology, Faculty of Medicine, Karadeniz Technical University, Mine Kadioglu, TR-61187 Trabzon, Turkey
N. I. Kalyoncu
Department of Pharmacology, Faculty of Medicine, Karadeniz Technical University, Mine Kadioglu, TR-61187 Trabzon, Turkey
E. Yaris
Department of Pharmacology, Faculty of Medicine, Karadeniz Technical University, Mine Kadioglu, TR-61187 Trabzon, Turkey