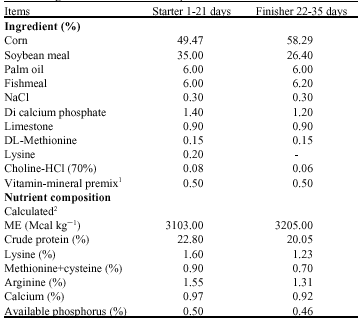
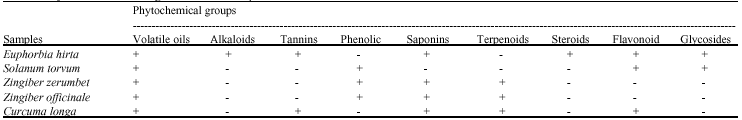
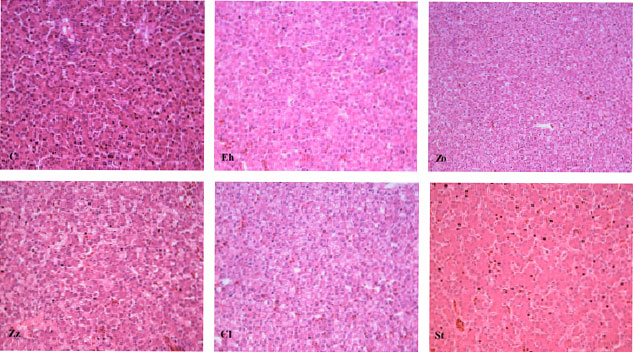
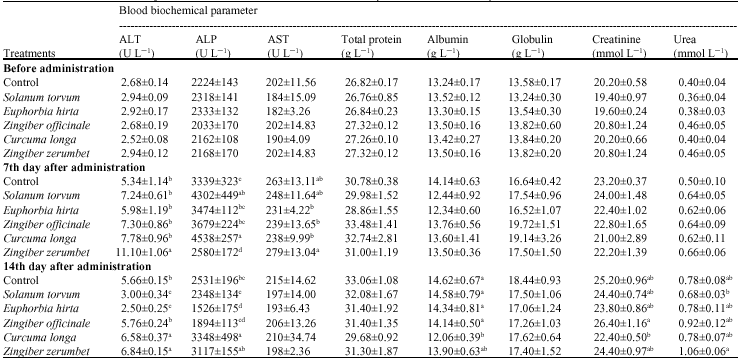
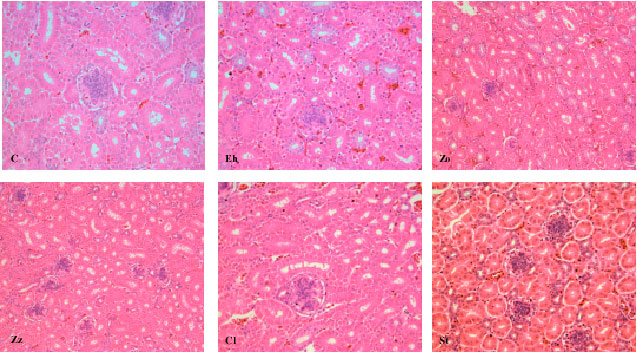
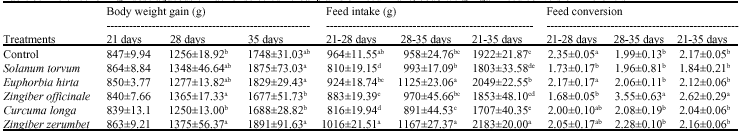
Research Article
Acute Toxicity Study and Phytochemical Screening of Selected Herbal Aqueous Extract in Broiler Chickens
Department of Animal Science
I. Zulkifli
Department of Animal Science
M. Hair Bejo
Department of Veterinary Pathology and Microbiology
A. Farida
Department of Food Sciences and Technology
M.N. Somchit
Department of Biomedical Sciences, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia