Research Article
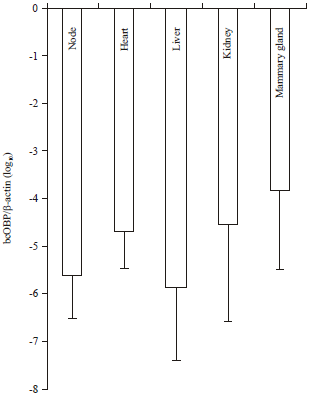
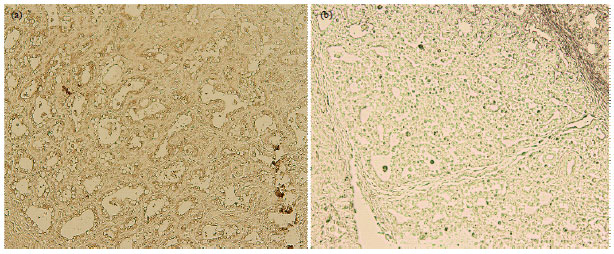
Quantification of Bovine Colostral Odorant-Binding Protein (bcOBP) mRNA Distributed in Principal Organs of Bovine Body
Department of Agriculture and Life Science, Obihiro University of Agriculture and Veterinary Medicine, 2-11 Inada-cho, Obihiro, Hokkaido, 080-8555, Japan
Tamar Japaridze
Department of Animal and Food Hygiene, Obihiro University of Agriculture and Veterinary Medicine, 2-11 Inada-cho, Obihiro, Hokkaido, 080-8555, Japan