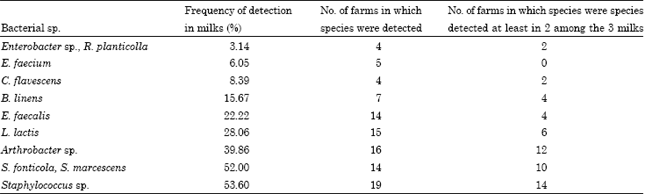
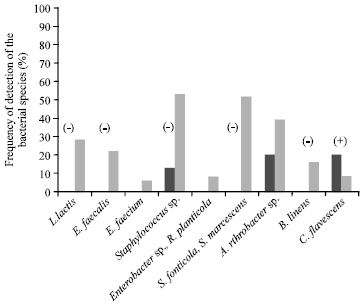
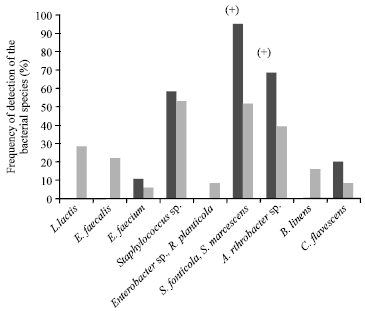
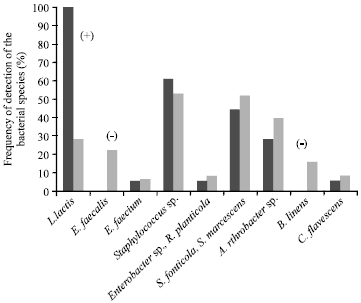
Research Article
Farm Management Practices and Diversity of the Dominant Bacterial Species in Raw Goat's Milk
Universite de Toulouse, Ecole d`Ingenieurs de Purpan, 75, voie du TOEC, BP 57611, F-31076 Toulouse Cedex 03, France
A. Delacroix-Buchet
INRA (UMR 1319) and AgroParisTech, Institut Micalis, F-78352 Jouy-en-Josas, France
C. Lopez
Institut de l`Elevage, 149 rue de Bercy-75595 Paris Cedex 12, France
D. Ali Haimoud Lekhal
Universite de Toulouse, Ecole d`Ingenieurs de Purpan, 75, voie du TOEC, BP 57611, F-31076 Toulouse Cedex 03, France
C. Roques
Universite de Toulouse; UPS, LU 49, Adhesion bacterienne et formation de biofilms, 35 chemin des maraichers, F-31062 Toulouse cedex 9, France