Research Article
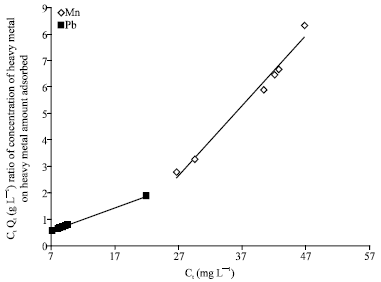
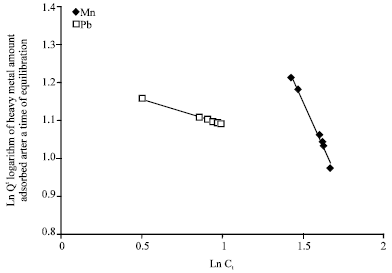
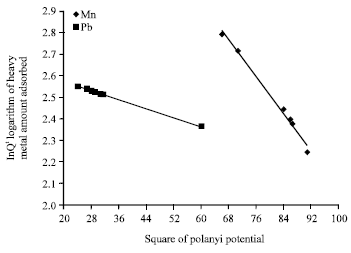
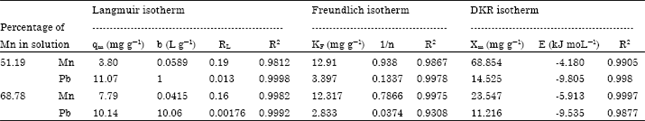
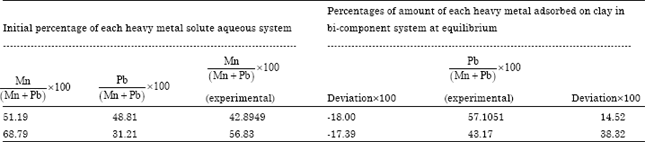
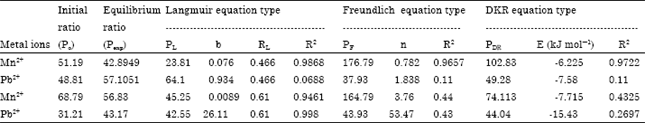
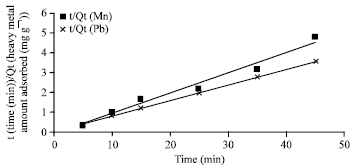
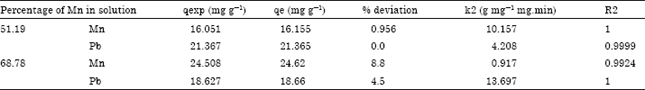
Isothermal and Kinetic Studies of Simultaneous Removal of Mn (II) and Pb (II) Ions in Aqueous Solution by Adsorption onto Clay from Bikougou Deposit (Gabon)
Laboratoire Pluridisciplinaire des Sciences de l�Ecole Normale Superieure de Libreville BP 17009, Libreville (Gabon)
J. Ndong Nlo
Laboratoire Pluridisciplinaire des Sciences de l�Ecole Normale Superieure de Libreville BP 17009, Libreville (Gabon)
Z.H. Moussambi Membetsi
Laboratoire Pluridisciplinaire des Sciences de l�Ecole Normale Superieure de Libreville BP 17009, Libreville (Gabon)
J.A. Ondo
Laboratoire Pluridisciplinaire des Sciences de l�Ecole Normale Superieure de Libreville BP 17009, Libreville (Gabon)
R. Kouya Biboutou
Laboratoire Pluridisciplinaire des Sciences de l�Ecole Normale Superieure de Libreville BP 17009, Libreville (Gabon)
P. Edou Engonga
Laboratoire Pluridisciplinaire des Sciences de l�Ecole Normale Superieure de Libreville BP 17009, Libreville (Gabon)