Research Article
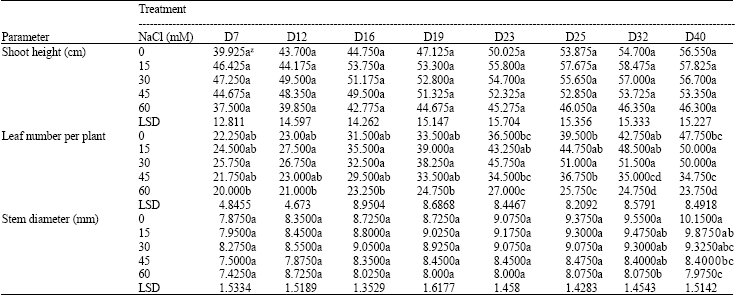
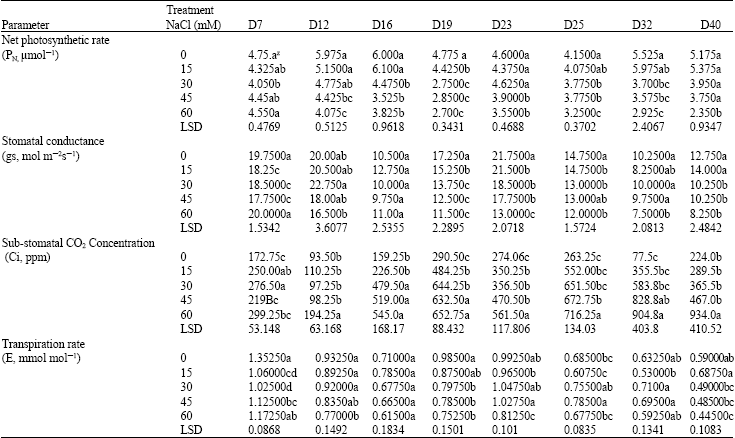
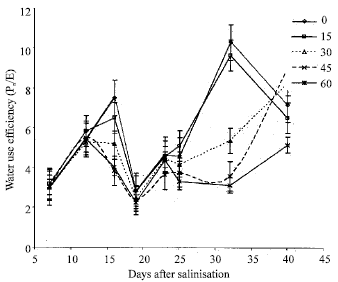
Effects of Salinity on Growth and Photosynthesis of Avocado Seedlings
Department of Botany and Horticulture, Maseno University, P.O. Box 333, Maseno, Kenya
LiveDNA: 254.15012
G. W. Netondo
Department of Botany and Horticulture, Maseno University, P.O. Box 333, Maseno, Kenya
G. Ouma
Department of Botany and Horticulture, Maseno University, P.O. Box 333, Maseno, Kenya