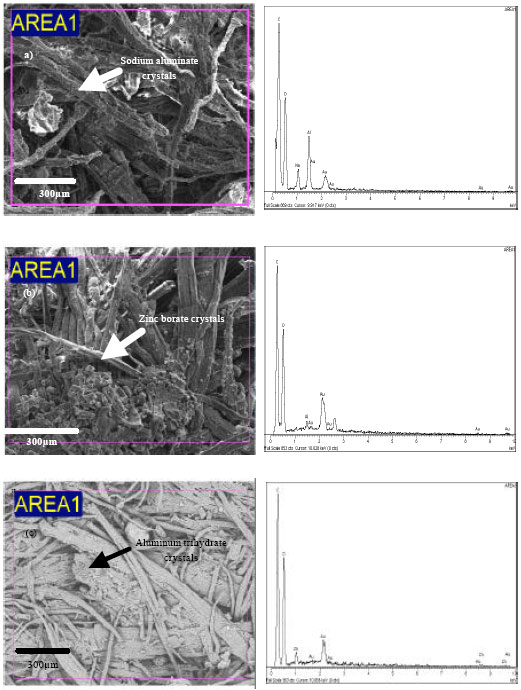
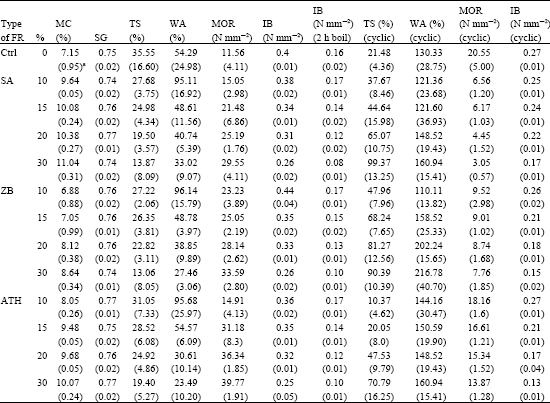
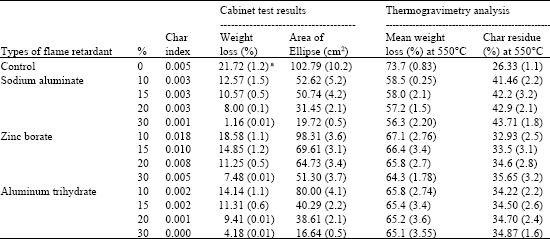
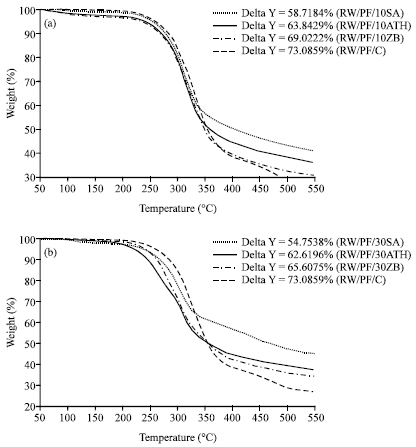
Research Article
Effect of Incorporation of Flame Retardants on Some of the Properties of Phenol Formaldehyde Medium Density Fiberboard
Div. Bio-resource, Paper and Coatings Technology, School of Industrial Technology, Universiti Sains Malaysia, 11800, Penang, Malaysia
R. Hashim
Department of Biological Science, Sir Alexander Fleming Building, Imperial College London SW7 2AZ, United Kingdom
R.N. Kumar
Div. Bio-resource, Paper and Coatings Technology, School of Industrial Technology, Universiti Sains Malaysia, 11800, Penang, Malaysia
P. Tamyez
Div. Bio-resource, Paper and Coatings Technology, School of Industrial Technology, Universiti Sains Malaysia, 11800, Penang, Malaysia
R.J. Murphy
Department of Biological Science, Sir Alexander Fleming Building, Imperial College London SW7 2AZ, United Kingdom
Z. Ali
School of Mathematical Science, Universiti Sains Malaysia 11800, Penang, Malaysia