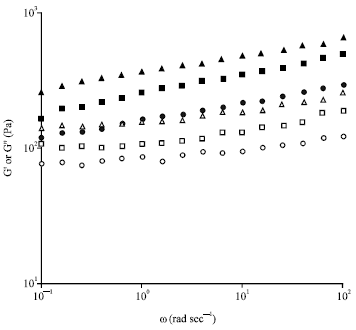
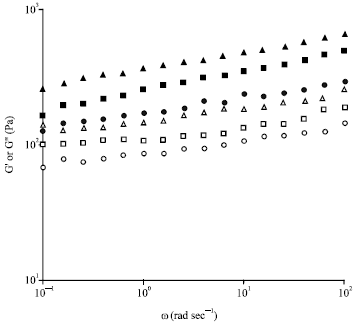
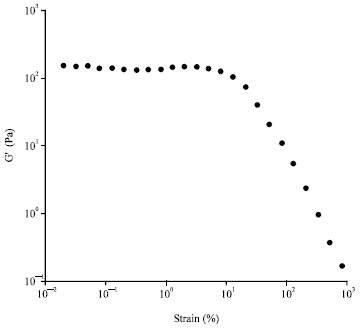
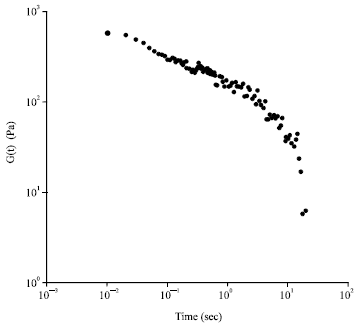
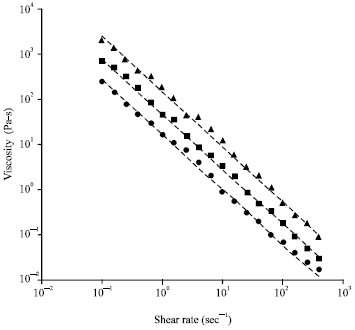
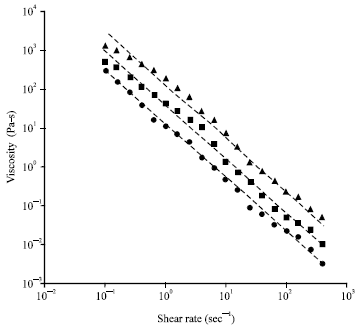
Research Article
Rheological Properties of Lupin Protein Suspensions
National Center for Agricultural Utilization Research, Agricultural Research Service, US Department of Agriculture, 1815 North University Street, Peoria, Illinois 61604, USA
Abdellatif A. Mohamed
National Center for Agricultural Utilization Research, Agricultural Research Service, US Department of Agriculture, 1815 North University Street, Peoria, Illinois 61604, USA
David J. Sessa
National Center for Agricultural Utilization Research, Agricultural Research Service, US Department of Agriculture, 1815 North University Street, Peoria, Illinois 61604, USA