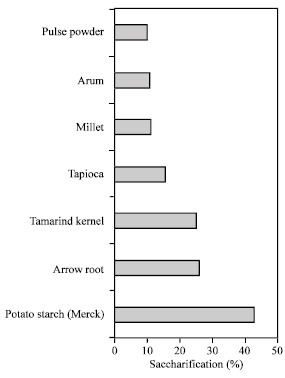
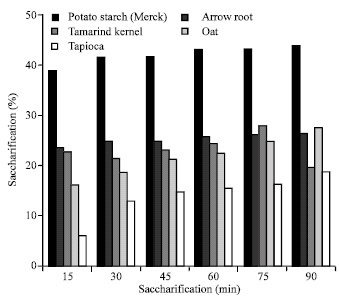
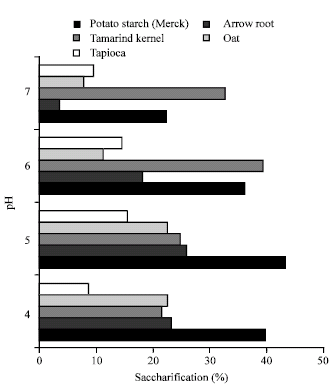
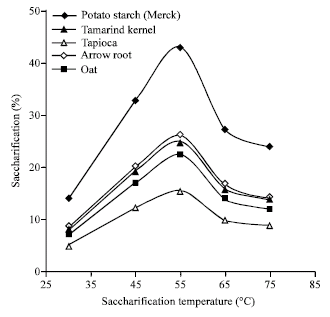
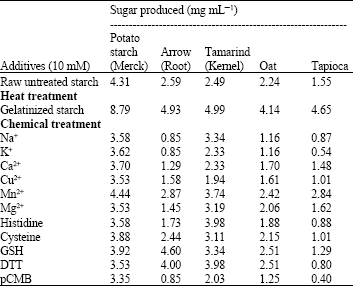
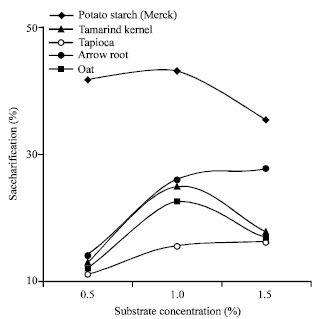
Research Article
Saccharification of Raw Native Starches by Extracellular Isoamylase of Rhizopus oryzae
Microbiology Research Laboratory, Department of Zoology, Molecular Biology and Genetics, Presidency College, 86/1, College Street, Kolkata 700 073, India
R.R. Ray
Microbiology Research Laboratory, Department of Zoology, Molecular Biology and Genetics, Presidency College, 86/1, College Street, Kolkata 700 073, India