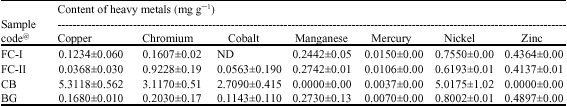
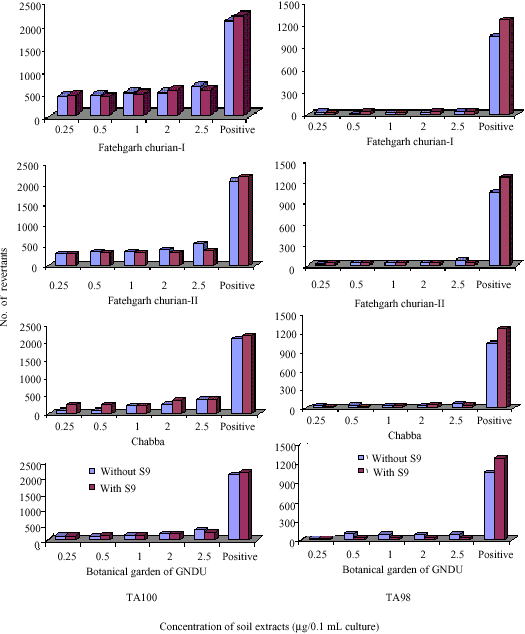
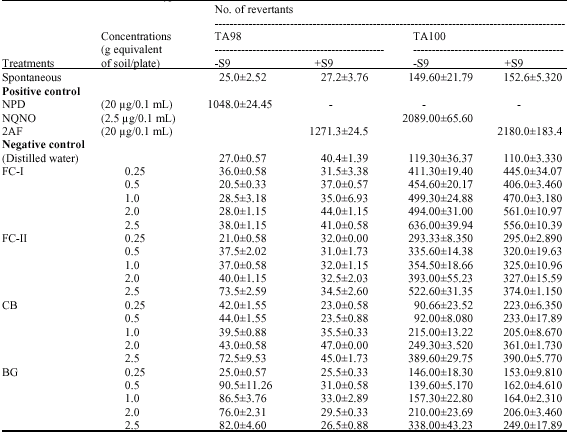
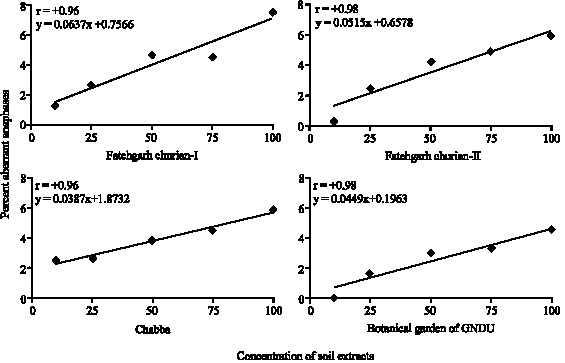
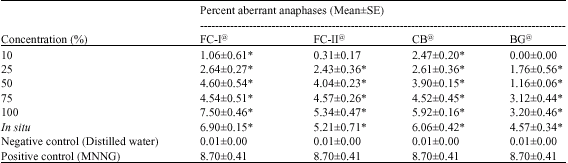
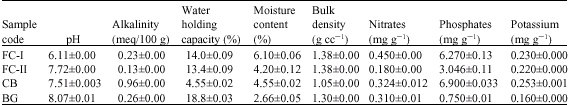
Research Article
Genotoxic Potential of Agricultural Soils of Amritsar
Department of Botanical and Environmental Sciences, Guru Nanak Dev University, Amritsar-143005, Punjab, India
S. Arora
Department of Botanical and Environmental Sciences, Guru Nanak Dev University, Amritsar-143005, Punjab, India
A. Nagpal
Department of Botanical and Environmental Sciences, Guru Nanak Dev University, Amritsar-143005, Punjab, India