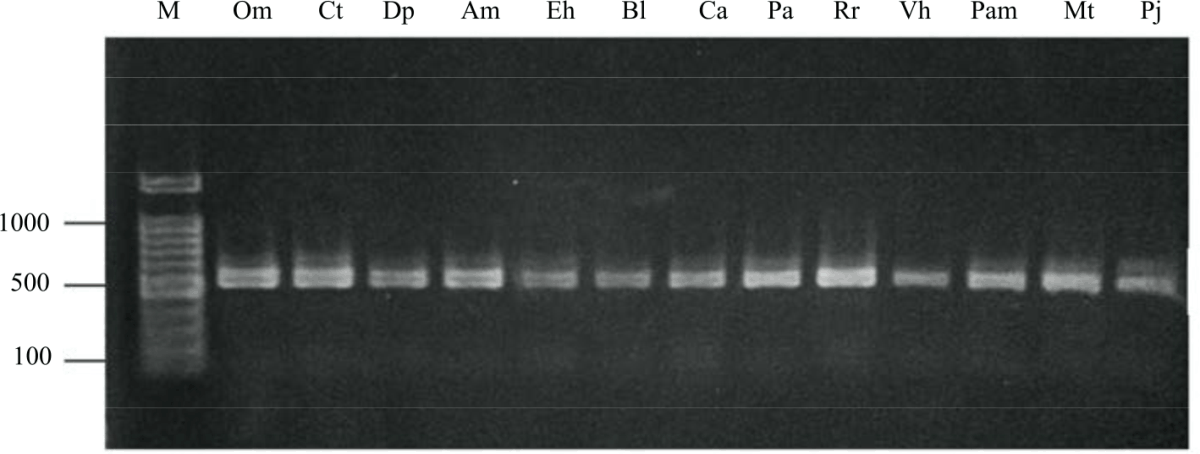
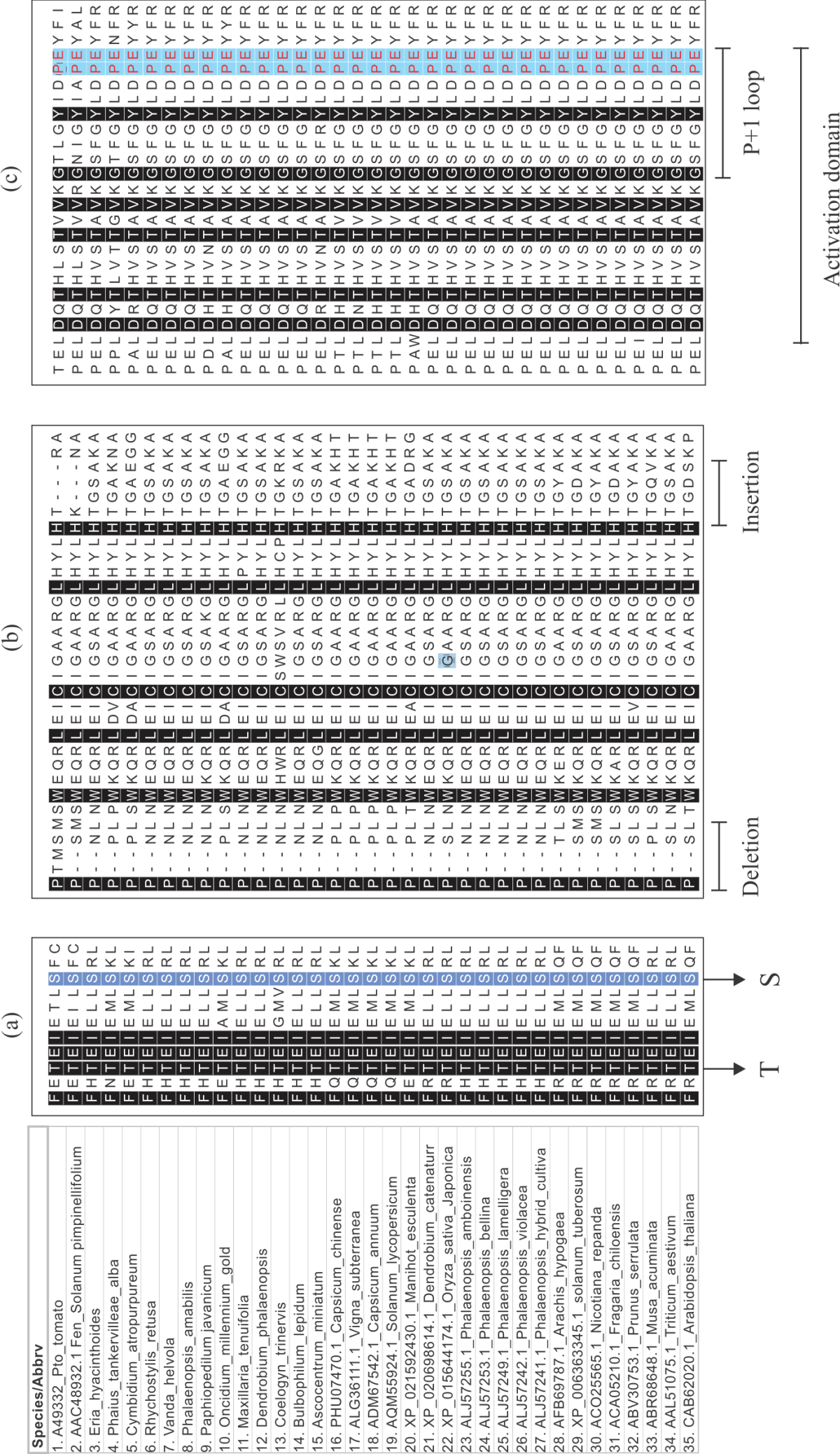
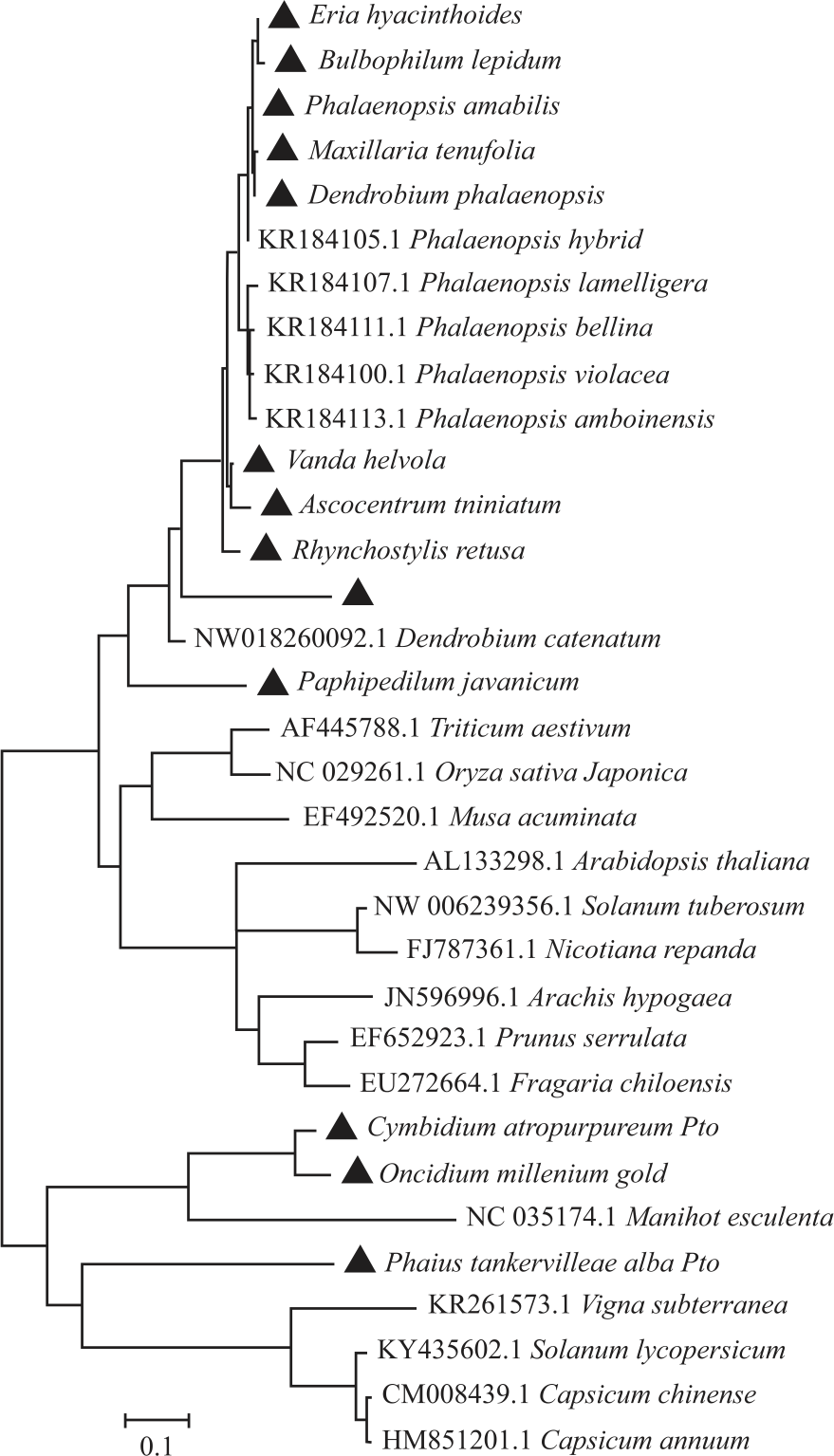
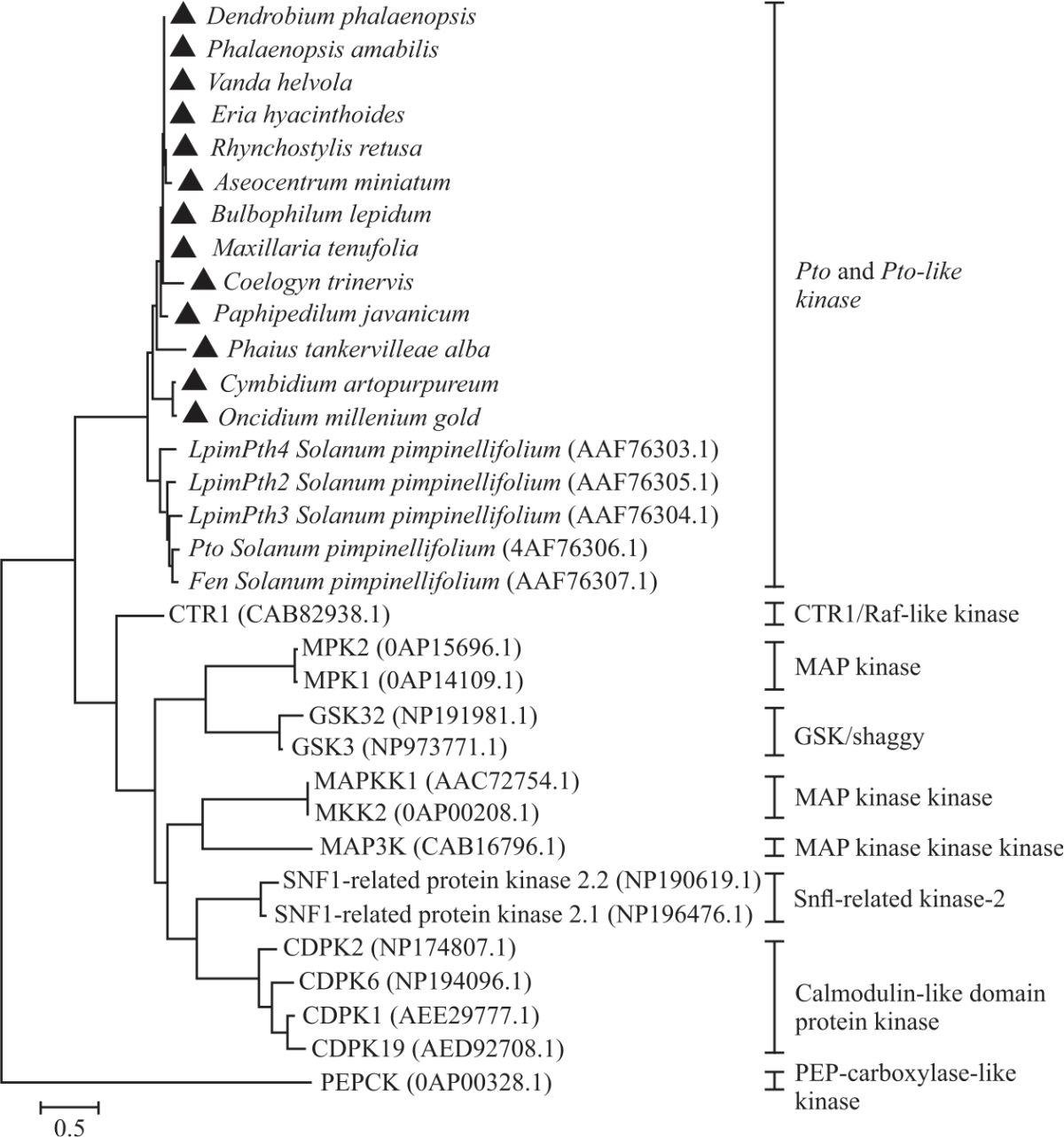
Research Article
Characterization of Pto-like Protein Kinase Disease Resistance Genes in Orchid
Department of Plant Protection, Faculty of Agriculture, Universitas Gadjah Mada, Flora Street No. 1 Bulaksumur, Yogyakarta 55281, Indonesia
Tri Joko
Department of Plant Protection, Faculty of Agriculture, Universitas Gadjah Mada, Flora Street No. 1 Bulaksumur, Yogyakarta 55281, Indonesia
LiveDNA: 62.16906