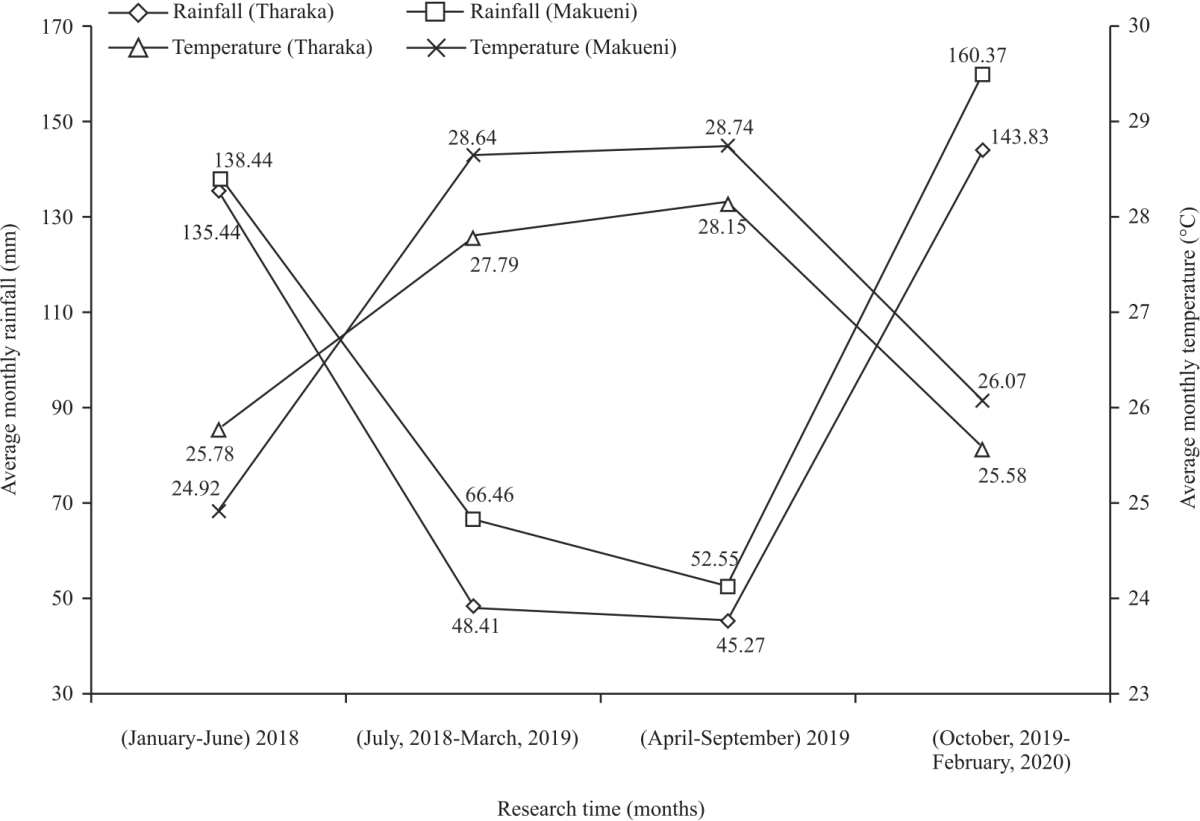
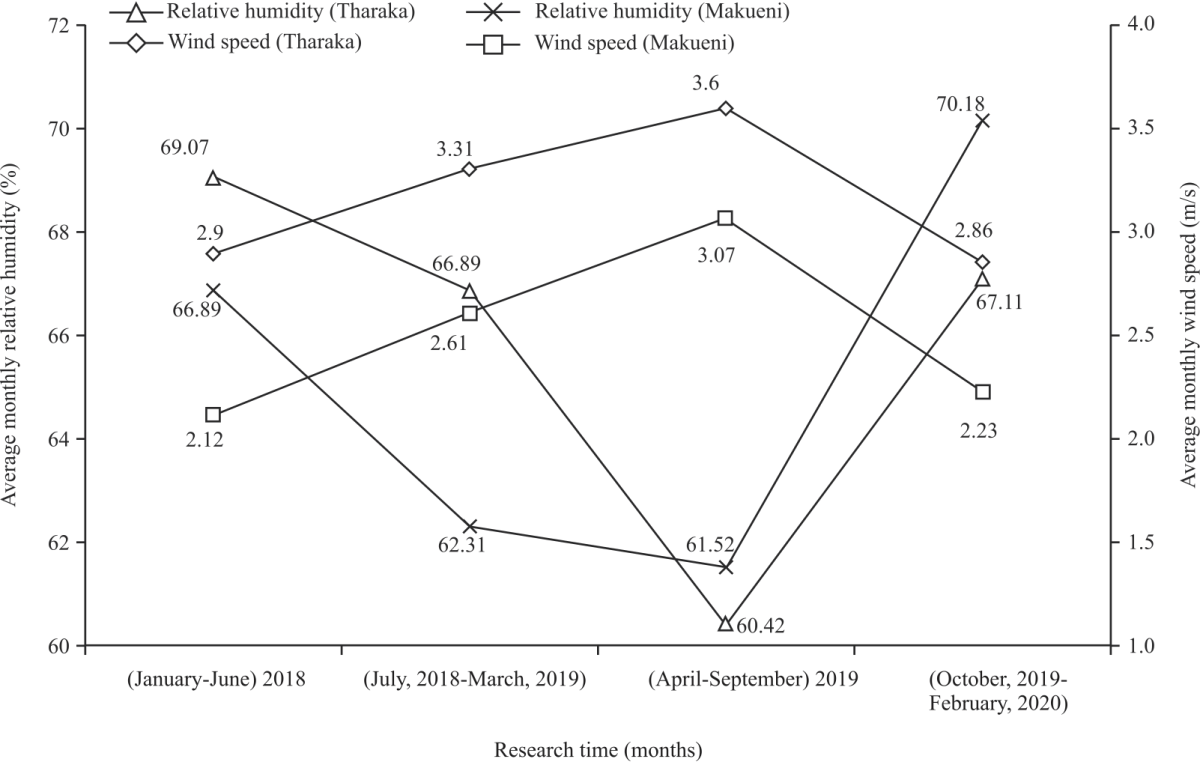
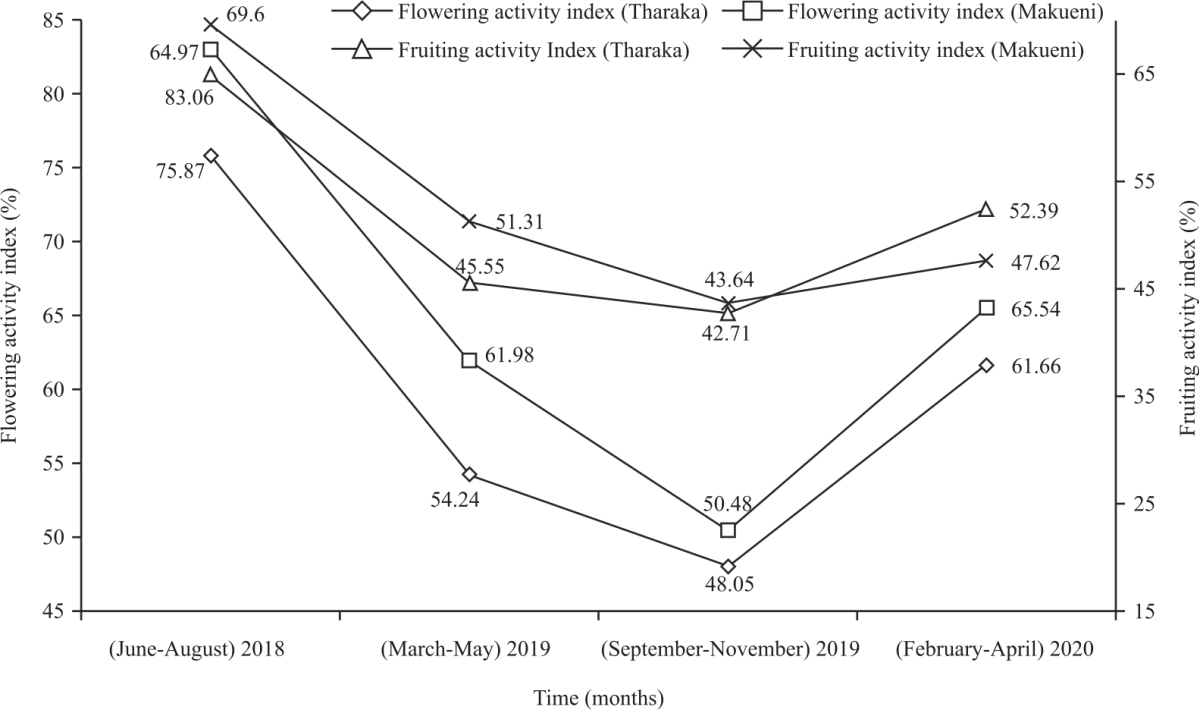
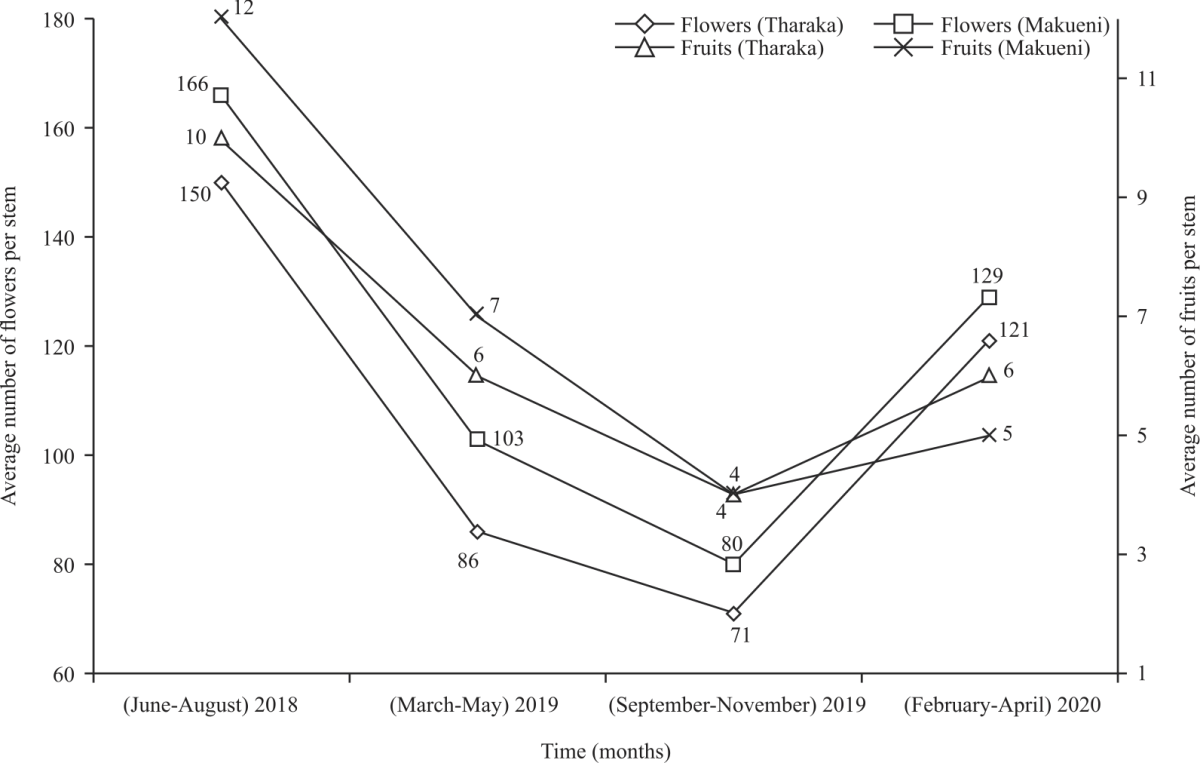
Research Article
Edaphic and Climatic Factors Affecting Phenology of Naturally Growing Calotropis procera in Semi-arid Regions of Kenya
School of Natural Resource Management, University of Eldoret, P.O. Box 1125-30100, Eldoret, Kenya
LiveDNA: 254.33730
Kenneth Odhiambo
School of Natural Resource Management, University of Eldoret, P.O. Box 1125-30100, Eldoret, Kenya
Alice Muchugi
World Agroforestry (ICRAF), P.O. Box 30677-00100, Nairobi, Kenya
Daniel Nyamai
School of Agriculture, Natural Resources and Environmental Studies, Rongo University, P.O. Box 103-40404, Rongo, Kenya
Agnes Gachuiri
World Agroforestry (ICRAF), P.O. Box 30677-00100, Nairobi, Kenya