Research Article
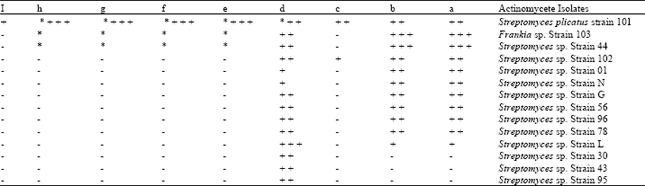
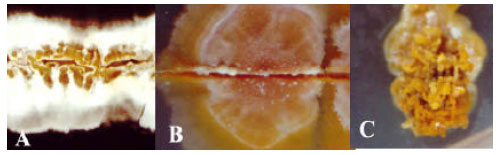
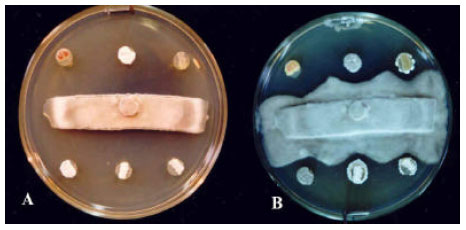
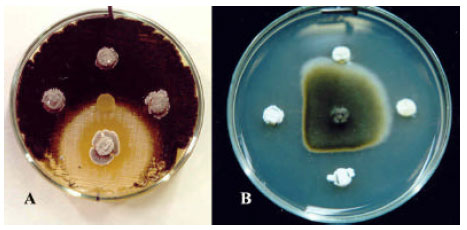
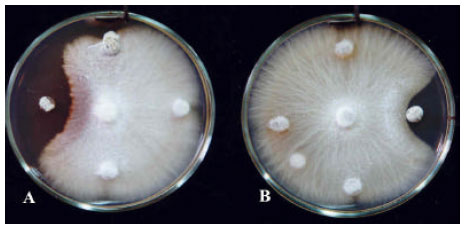
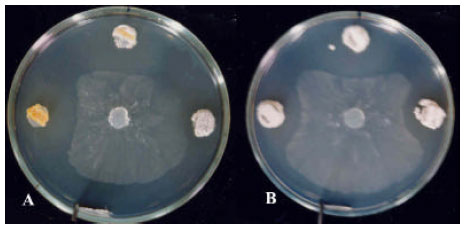
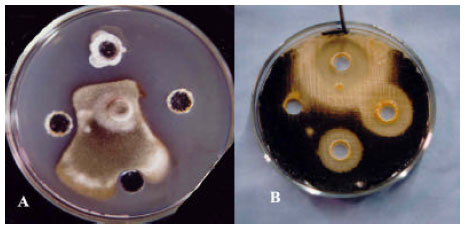
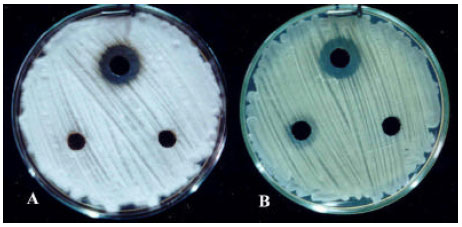
First Report of Antifungal Spectra of Activity of Iranian Actinomycetes Strains Against Alternaria solani, Alternaria alternate, Fusarium solani, Phytophthora megasperma, Verticillium dahliae and Saccharomyces cerevisiae
Not Available
G.H. Shahidi Bonjar
Not Available
R. Rawashdeh
Not Available
S. Batayneh
Not Available
I. Saadoun
Not Available