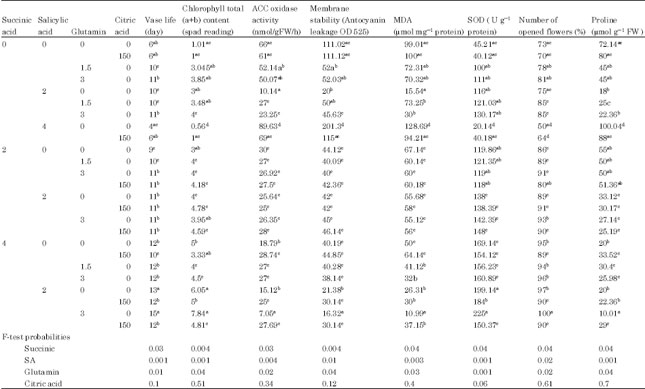
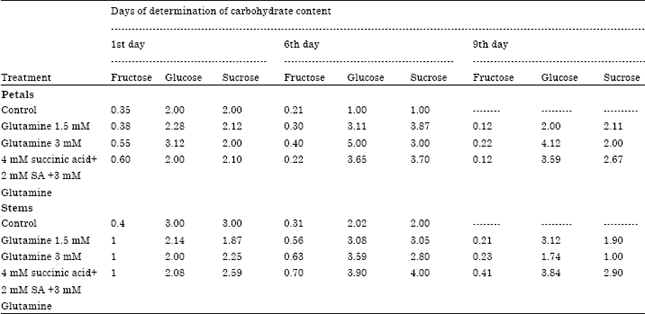
Research Article
Extending the Vase Life of Lisianthus (Eustoma grandiflorum Mariachii. cv. blue) with Different Preservatives
Department of Horticulture Sciences, Faculty of Agriculture and Natural Resources, Islamic Azad University, Karaj Branch, Young Researchers Club, Karaj, Iran
M. Aran
Department of Landscape, College of Agriculture, University of Zabol, Zabol, Iran
S. Zamani
Department of Horticulture Sciences, Faculty of Agriculture and Natural Resources, Islamic Azad University, Karaj Branch, Young Researchers Club, Karaj, Iran