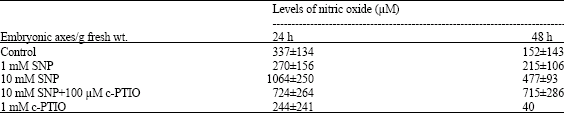
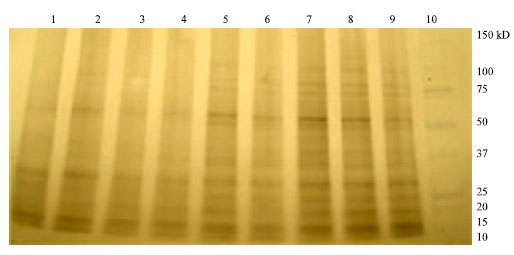
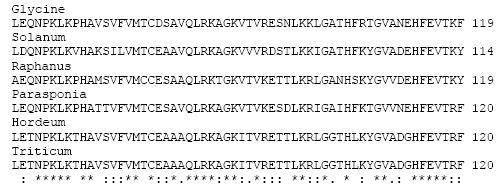Research Article
S-Nitrosylation Process Acts as a Regulatory Switch for Seed Germination in Wheat
Department of Biology, School of Science, Engineering and Mathematics,Bethune Cookman University, 640 Dr. Mary McLeod Bethune Boulevard,Daytona Beach, Florida 32114-3099, USA