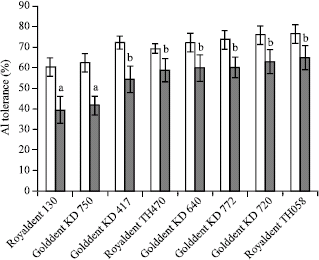
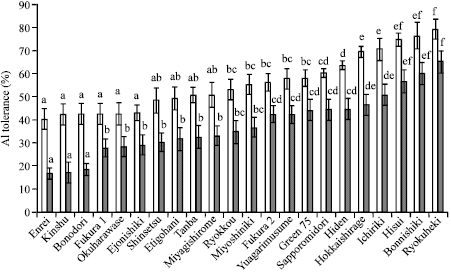
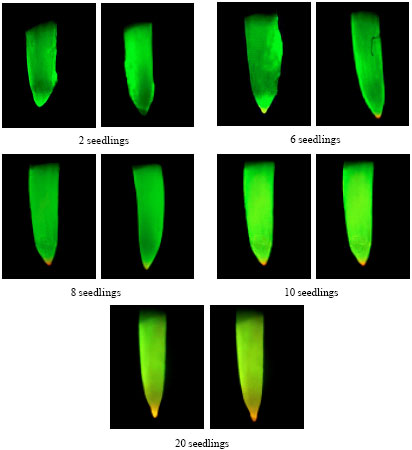
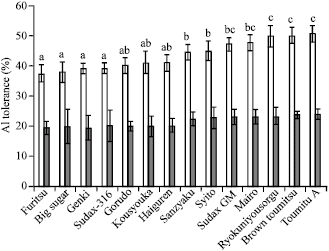
Research Article
Comparative Studies on Aluminum Tolerance Screening Techniques for Sorghum, Soybean and Maize in Simple Solution Culture
Laboratory of Plant Nutrition and Soil Science, Faculty of Agriculture, Yamagata University, 1-23 Wakaba Machi, Tsuruoka, Yamagata 997 8555, Japan
T. Wagatsuma
Laboratory of Plant Nutrition and Soil Science, Faculty of Agriculture, Yamagata University, 1-23 Wakaba Machi, Tsuruoka, Yamagata 997 8555, Japan
M.S.H. Khan
Laboratory of Plant Nutrition and Soil Science, Faculty of Agriculture, Yamagata University, 1-23 Wakaba Machi, Tsuruoka, Yamagata 997 8555, Japan
K. Tawaraya
Laboratory of Plant Nutrition and Soil Science, Faculty of Agriculture, Yamagata University, 1-23 Wakaba Machi, Tsuruoka, Yamagata 997 8555, Japan