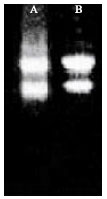
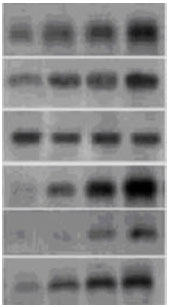
Research Article
Isolation of cDNA Clones of Genes Differentially Expressed During Somatic Embryogenesis of Pinus roxburghii
Division of Plant Biotechnology, Department of Botany, Karnatak University, Pavate Nagar, Dharwad-580003, Karnataka State, India
K. Nataraja
Division of Plant Biotechnology, Department of Botany, Karnatak University, Pavate Nagar, Dharwad-580003, Karnataka State, India