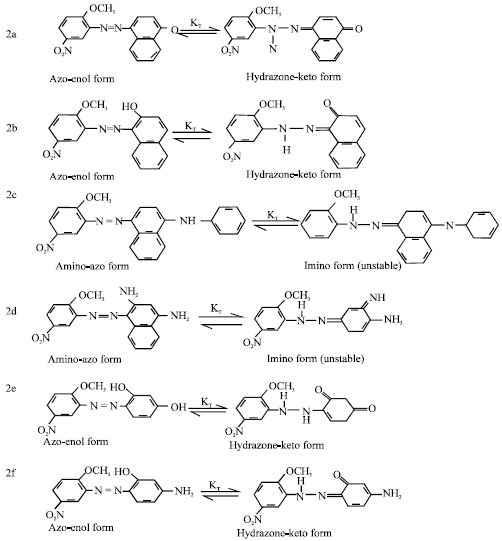
Research Article
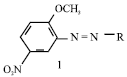
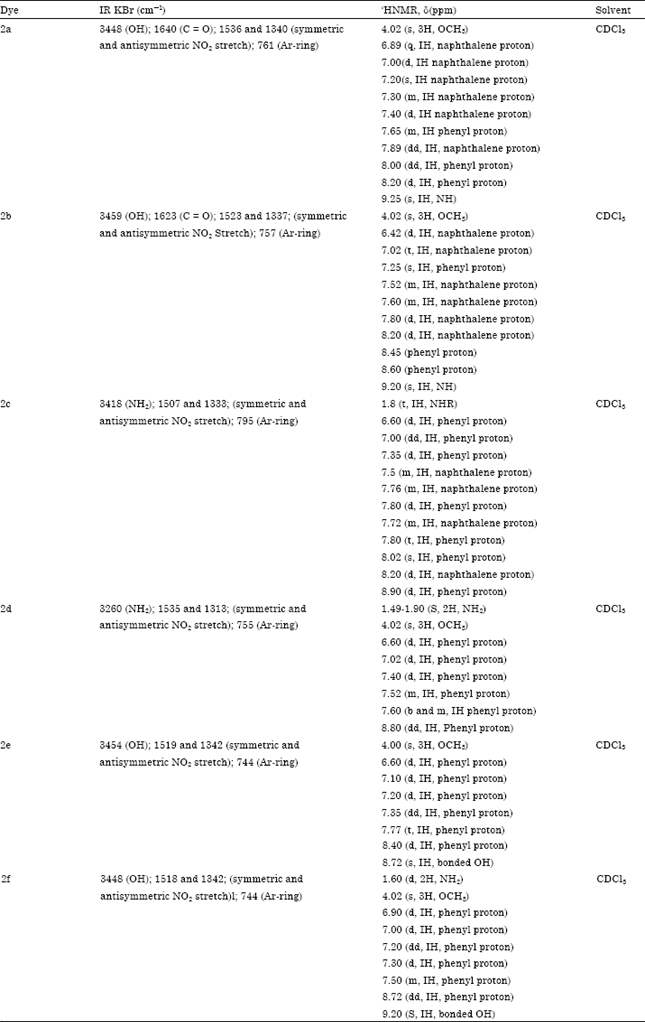
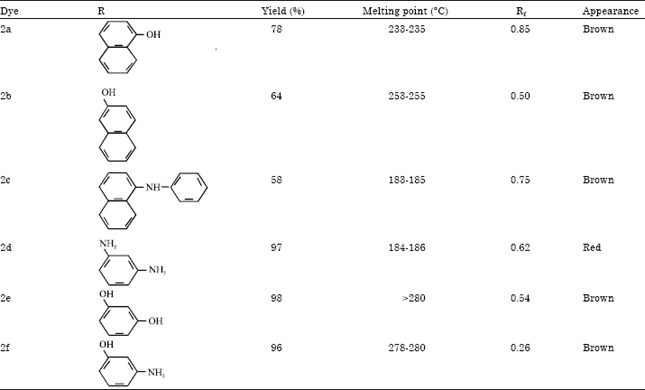
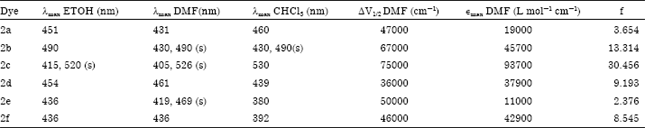
Synthesis and Absorption Spectra of Monoazo Dyes derived from 2-Methoxy-5-Nitroaniline
Department of Chemistry, Faculty of Science, Delta State University, P.M.B. 1, Abraka, Delta State, Nigeria
E. Osabohien
Department of Chemistry, Faculty of Science, Delta State University, P.M.B. 1, Abraka, Delta State, Nigeria