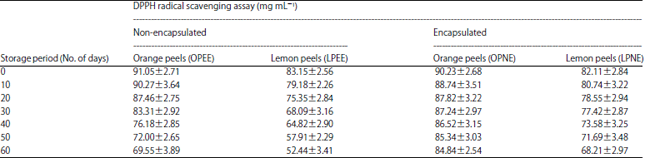
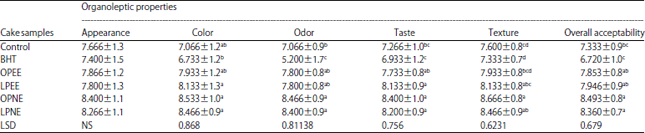
Research Article
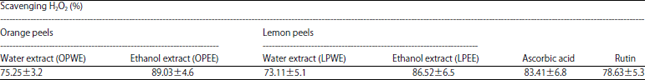
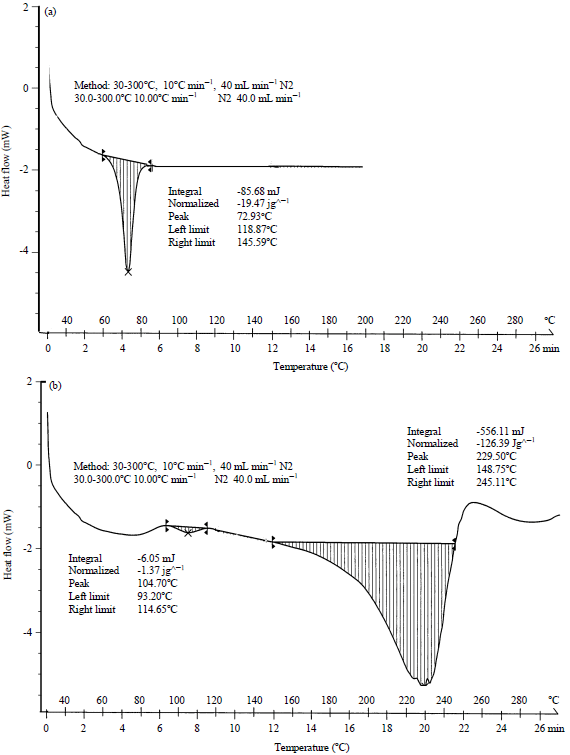
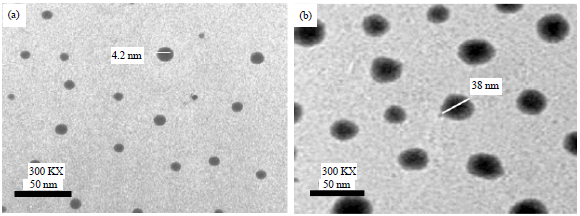
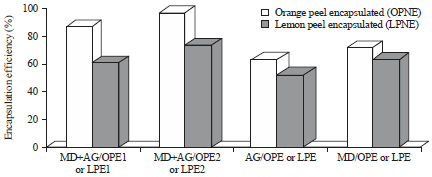
Nano-encapsulation Efficiency of Lemon and Orange Peels Extracts on Cake Shelf Life
Department of Food Technology, National Research Centre, Egypt
LiveDNA: 20.13002
Mona A. Ibrahim
Department of Food Technology, National Research Centre, Egypt
El-Demery Mervat
Department of Home Economic, Faculty of Specific Education, Kafrelsheikh University, Egypt
Hamdy A. Shaaban
Department of Chemistry of Flavour and Aroma, National Research Centre, Egypt
LiveDNA: 20.16477
Mohie M. Kamil
Department of Food Technology, National Research Centre, Egypt
Nefisa A. Hegazy
Department of Food Technology, National Research Centre, Egypt