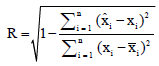Research Article
Prediction of Total Phenolics and Flavonoids Contents in Chinese Wild Rice (Zizania latifolia) Using FT-NIR Spectroscopy
College of Food Science and Engineering, Northwest A and F University, Yangling, China
Mingtao Fan
College of Food Science and Engineering, Northwest A and F University, Yangling, China
Tingjing Zhang
College of Food Science and Engineering, Northwest A and F University, Yangling, China
Kun Yang
College of Food Science and Engineering, Northwest A and F University, Yangling, China









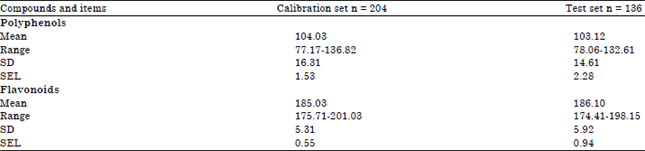
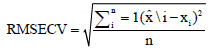
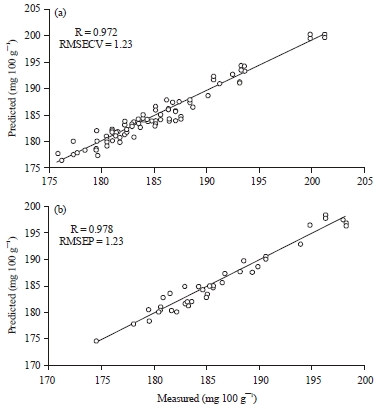
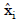 is the predicted value of the model for test sample and is the number of samples in the test set.
is the predicted value of the model for test sample and is the number of samples in the test set.