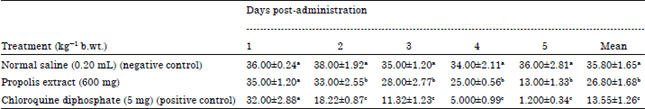
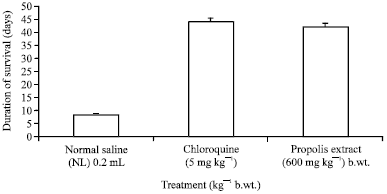
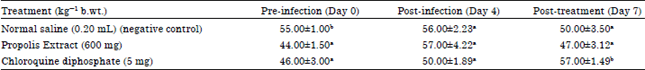
Research Article
Therapeutic Potentials of Nigerian Insect-propolis Against the Malarial Parasite, Plasmodium berghei (Haemosporida: Plasmodidae)
Department of Biological Sciences, Federal University of Technology, Minna, P.M.B. 65, Minna, Niger State, Nigeria