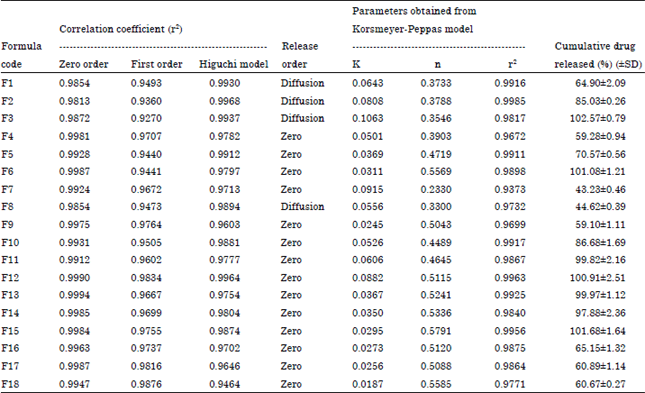
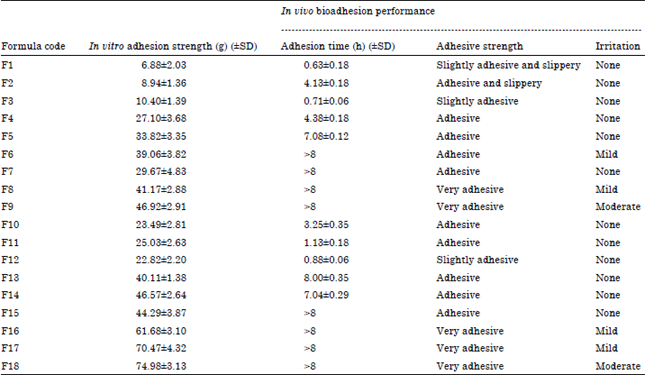
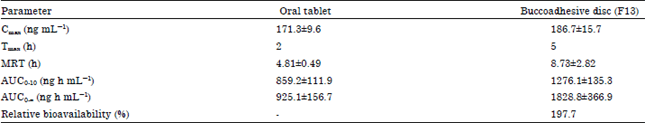
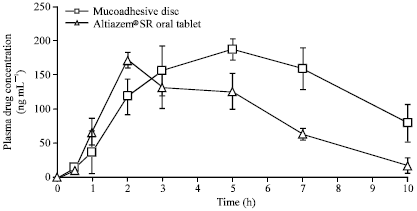
Research Article
Formulation and In vitro/In vivo Evaluation of Buccoadhesive Discs for Controlled Release of Calcium Channel Antagonist
Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmacy, Cairo University, Cairo, Egypt
Magdy Mohamed
Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmacy, Cairo University, Cairo, Egypt
Muaadh Ali
Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmacy, Cairo University, Cairo, Egypt