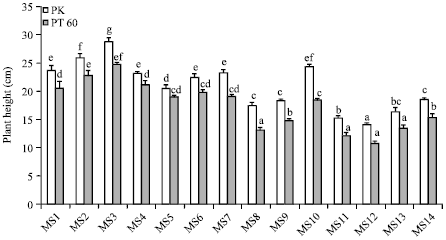
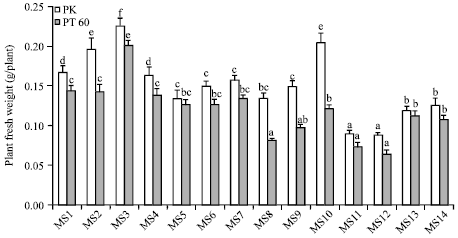
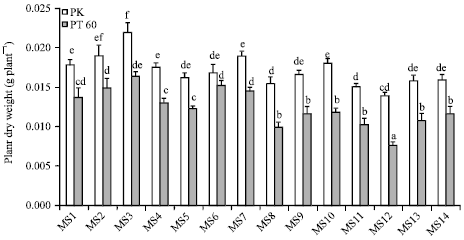
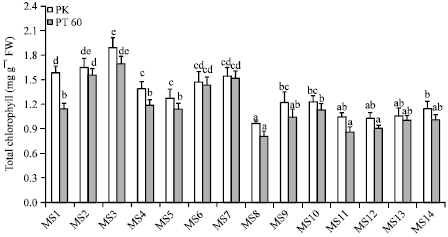
Research Article
Alleviation of Adverse Effects of Salt Stress on Rice Seedlings by Exogenous Trehalose
Department of Biology, Faculty of Science, Khon Kaen University, Khon Kaen 40002, Thailand
S. Phongngarm
Department of Biology, Faculty of Science, Khon Kaen University, Khon Kaen 40002, Thailand