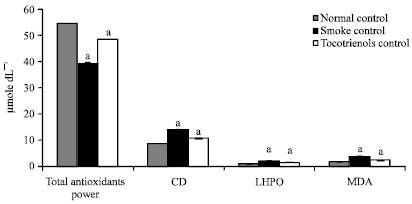
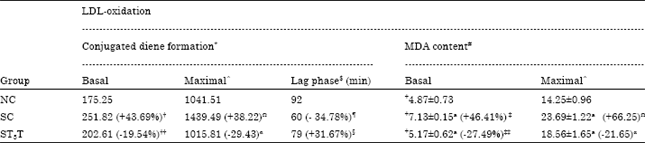
Research Article
Effect of Dietary Tocotrienols on Antioxidant Status and Low Density Lipoprotein Oxidation in Rats, Exposed to Cigarette Smoke
Department of Biochemistry, Division of Life science, Sardar Bhagwan Singh Post Graduate, Institute of Biomedical Sciences and Research Balawala, 248001, Dehradun, UK, India
Fouzia Ishaq
Department of Zoology and Environmental Science, Gurukula Kangri University, Haridwar-249244, UK, India
Abhay S. Chandel
Department of Biotechnology, HNB Garhwal University, Srinagar, Uttarakhand, India
Samir Chettri
Department of Biotechnology, HNB Garhwal University, Srinagar, Uttarakhand, India