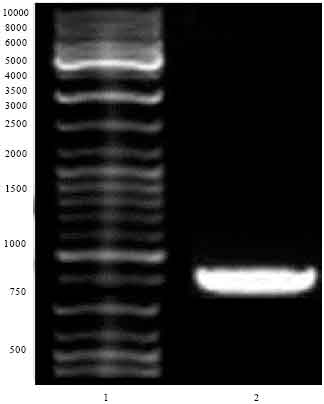
Research Article
Expression of Complete Rhoptry Protein 1 (ROP1) Gene of Toxoplasma gondii in Eukaryotic Cell
Department of Biology, Sanandaj Branch, Islamic Azad University, Sanandaj, Iran
Parviz Ashtari
Agriculture, Medicine and Industrial Research School, Nuclear Science and Technology Research Institute, Karaj, Iran