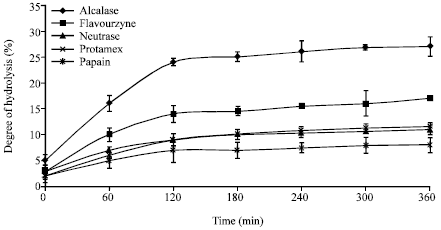
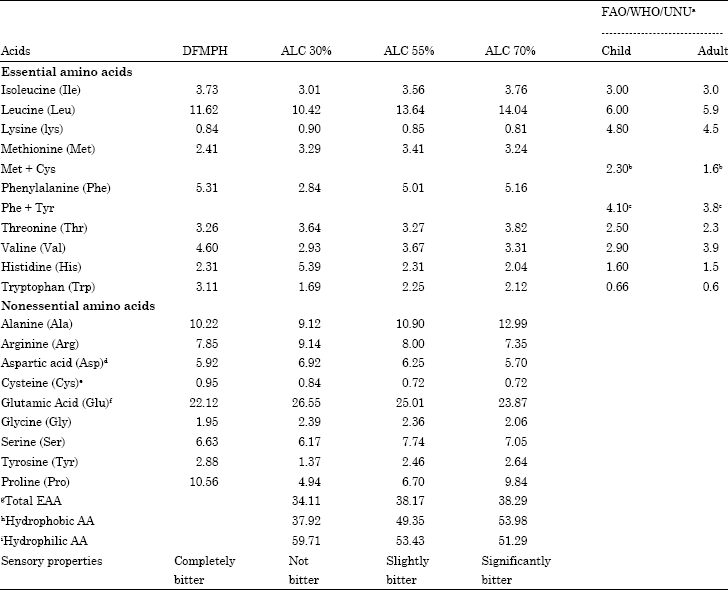
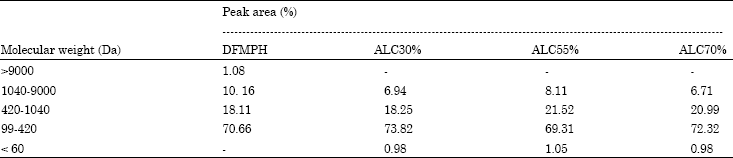
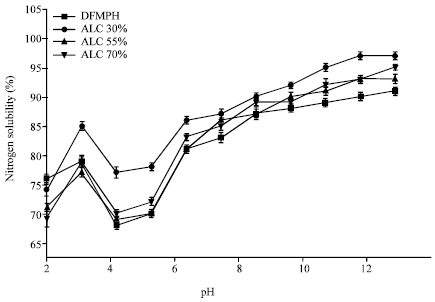
Research Article
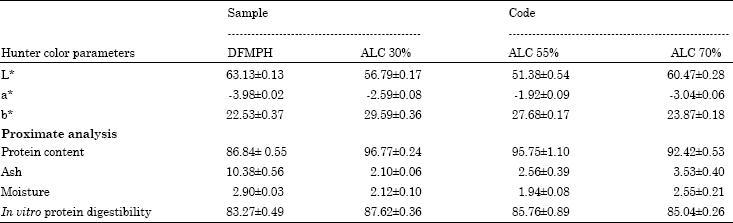
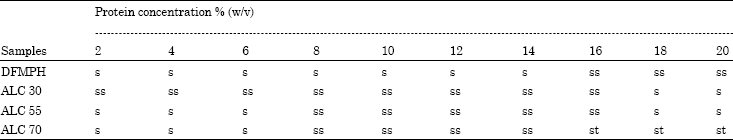
The Influence of Debittering and Desalting on Defatted Foxtail Millet (Setaria italica L.) Protein Hydrolysate
State Key Laboratory of Food Science and Technology, Jiangnan University, No. 1800, Lihu Road, Wuxi, 214122 Jiangsu, China
I. Amadou
State Key Laboratory of Food Science and Technology, Jiangnan University, No. 1800, Lihu Road, Wuxi, 214122 Jiangsu, China
Zhu Kexue
State Key Laboratory of Food Science and Technology, Jiangnan University, No. 1800, Lihu Road, Wuxi, 214122 Jiangsu, China
M.B. Kelfala Foh
State Key Laboratory of Food Science and Technology, Jiangnan University, No. 1800, Lihu Road, Wuxi, 214122 Jiangsu, China
Z. Huiming
State Key Laboratory of Food Science and Technology, Jiangnan University, No. 1800, Lihu Road, Wuxi, 214122 Jiangsu, China