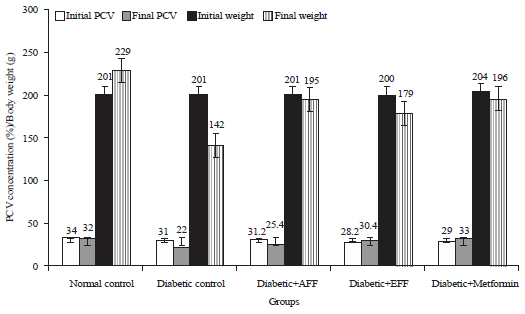
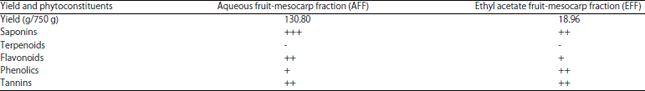
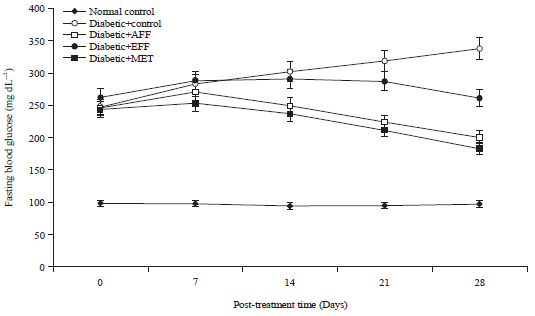
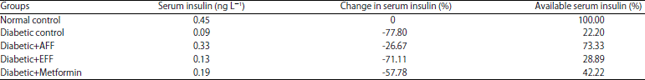
Research Article
Fractionated Ethanol Extract of Balanites aegyptiaca Fruit-Mesocarp Effect on Diabetes Mellitus Model Rats
Department of Medical Biochemistry, Abubakar Tafawa Balewa University, Bauchi, Nigeria
LiveDNA: 234.16428
Janet O. Alegbejo
Department of Pediatrics, Ahmadu Bello University Teaching Hospital, Zaria, Nigeria
Stanley I.R. Okoduwa
Directorate of Research and Development, Nigerian Institute of Leather and Science Technology, Zaria, Nigeria
LiveDNA: 234.30244
Kola M. Anigo
Department of Biochemistry, Ahmadu Bello University, Zaria, Nigeria