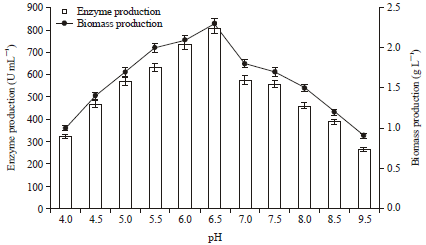
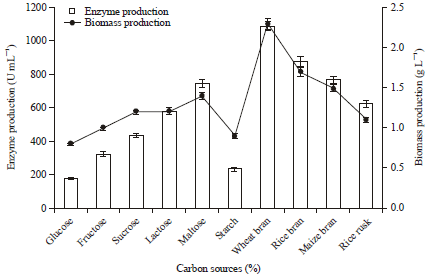
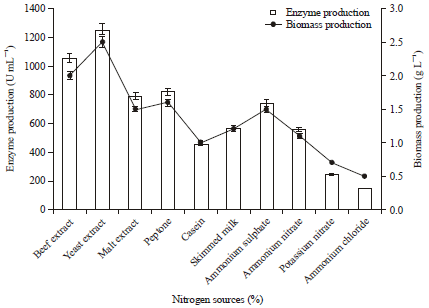
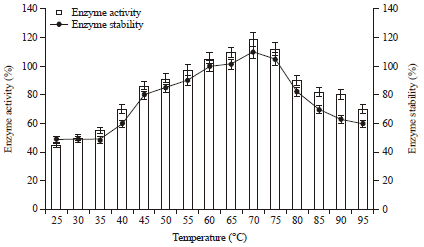
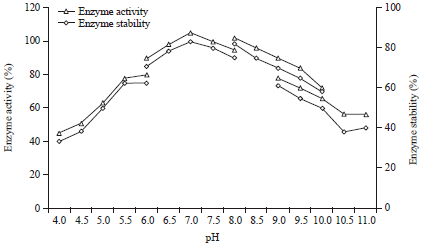
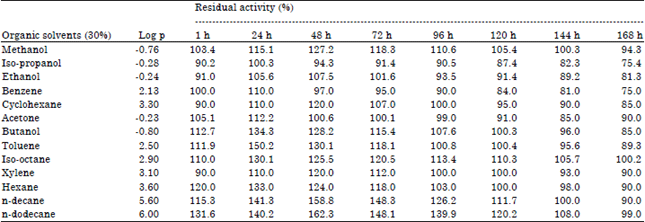
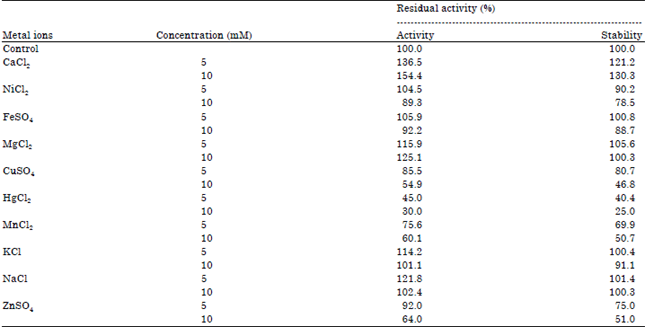
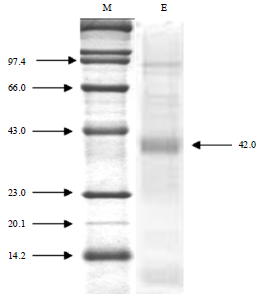
Research Article
Production and Characterization of Thermotolerant-Organic Solvent Resistant Acidic Protease by Pseudomonas aeruginosa RGSS-09 Isolated from Dairy Sludge
Department of Microbiology (Centre of Excellence), Dr. Ram Manohar Lohia Avadh University, Faizabad, 224001, Uttar Pradesh, India
Soni Tiwari
Department of Microbiology (Centre of Excellence), Dr. Ram Manohar Lohia Avadh University, Faizabad, 224001, Uttar Pradesh, India
Sheffalika Singh
Department of Microbiology (Centre of Excellence), Dr. Ram Manohar Lohia Avadh University, Faizabad, 224001, Uttar Pradesh, India