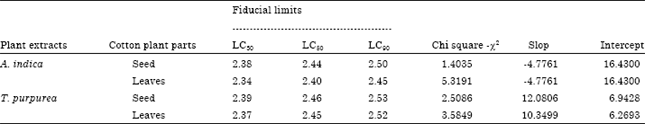
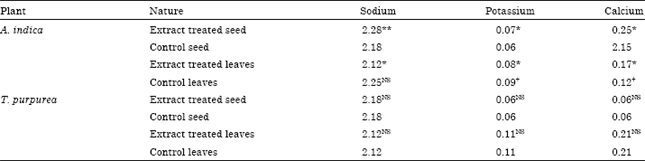
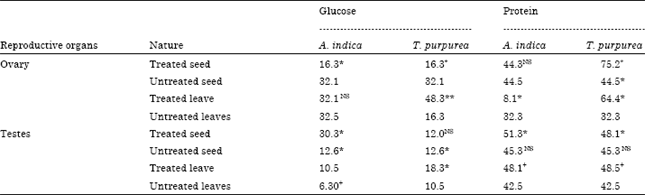
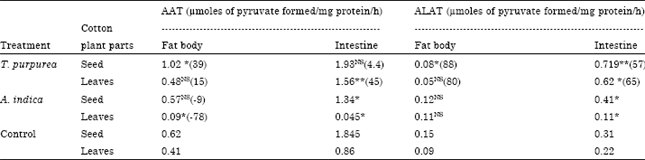
Research Article
Toxic Effect of Tephrosia purpurea (Linn.) and Acalypha indica (Linn.) Aqueous Extracts Impact on the Mortality, Macromolecules, Intestinal Electrolytes and Detoxication Enzymes of Dysdercus cingulatus (Fab.)
Department of Advanced Zoology and Biotechnology, Crop Protection Research Centre, St. Xavier`s College (Autonomous), Palayamkottai-627002, Tamil Nadu, India
J. Shoba
Department of Advanced Zoology and Biotechnology, Crop Protection Research Centre, St. Xavier`s College (Autonomous), Palayamkottai-627002, Tamil Nadu, India