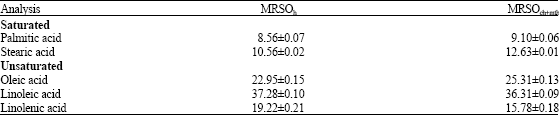
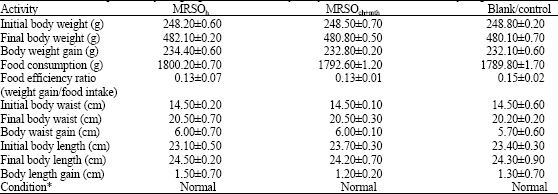
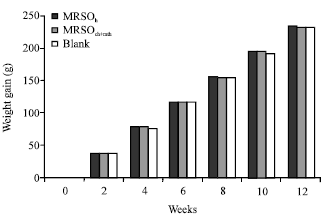
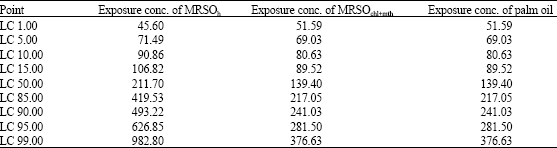
Research Article
Toxicity Study of Malaysian Rubber (Hevea brasiliensis) Seed Oil as Rats and Shrimps Tests
School of Chemical Sciences and Food Technology, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, 43600 Bangi, Selangor, Malaysia
Jumat Salimon
School of Chemical Sciences and Food Technology, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, 43600 Bangi, Selangor, Malaysia