Research Article
Molecular Modelling Analysis of the Metabolism of Enrofloxacin
School of Biomedical Sciences, Faculty of Health Sciences, C42, The University of Sydney, P.O. Box. 170, Lidcombe, NSW 1825, Australia
Fluoroquinoline antibiotics are increasingly used in veterinary medicine because of effectiveness in treating a wide range of bacterial infections (Bauditz, 1990). Fluoroquinolines have been used to successfully treat Gram positive, Gram negative and Mycoplasma such as pulmonary infections, urinary and digestive infections (Bauditz, 1990). A notable example of fluoroquinoline is enrofloxacin (ENFLX), 1-cyclopropyl-7-(4-ethyl-1-piperazinyl)-6-fluoro-1,4-dihydro-4-oxo-3-quinoline carboxylic acid that has been approved for use in veterinary medicine including food animals. ENFLX is used in cattle, pigs, poultry, fish, dogs and cats (Choma et al., 2004). It is effective against organisms that are resistant to the commonly used antibacterial substances such as tetracyclines and macrolides (Scheer, 1990; Prescott and Yelding, 1990). Ciprofloxacin [1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinoline carboxylic acid] (abbreviated as CPFLX), the major metabolite of ENFLX formed from its N-deethylation is also highly active (Anadon et al., 2001). CPFLX is used in both humans and animals and is one of the most popular antibiotics in the world. Because of its greater use in humans, CPFLX may be considered as human counterpart of ENFLX. The bactericidal activity of ENFLX and CPFLX, similar to that of other quinolines, is mediated by inhibition of bacterial DNA topoisomerase (Wang and Xie, 2005; Cozarelli, 1980; Anadon and Martinez, 1992). ENFLX is rapidly absorbed from the site of administration and well distributed into tissues (Ehinger et al., 2002).
Although fluoroquinolines are considered to be well-tolerated drugs, they may display a number of side-effects such as neurotoxicity, phototoxicity cardiotoxicity and hepatotoxicity and have the potential to induce Achilles tendon disorders such as tendinitis or even ruptures. It should also be noted that the intensive use of antibiotics in generals and fluoroquinolines in particular, in humans and animals, has led to a significant increase in antimicrobial resistance and therefore important consequences on human health (Tollefson and Miller 2002; Kennedy et al., 2000).
As stated earlier, the major metabolite of ENFLX is CPFLX which is highly active. It is a moderately potent and selective inhibitor of CYP1A2-mediated drug metabolism (Davis et al., 1996; Bertz and Granneman, 1997). CPFLX is further metabolized to produce four other metabolites namely formyl-ciprofloxacin (FCPFLX), oxociprofloxacin (OCPFLX), desethylciprofloxacin (DECPFLX) and sulfociprofloxacin (SCPFLX) (Anadon et al., 2001). In humans, all the four metabolites of CPFLX namely FCPFLX, OCPFLX, DECPFLX and SCPFLX have been detected in both urine and faeces (Fig. 1). In this study, molecular modelling analyses have been carried out using the program Spartan’02 [Spartan ‘02] to investigate the relative stability of ENFLX and its metabolites with the aim of providing a better understanding on the relative toxicity due to ENFLX and its metabolites. The work was carried out in the School of Biomedical Sciences, The University of Sydney during February to April 2006.
Computational Methods
The geometries of ENFLX and its metabolites CPFLX, OCPFLX, DECPFLX, FCPFLX and SCPFLX have been optimised based on molecular mechanics (Fig. 1), semi-empirical and DFT calculations, using the molecular modelling program Spartan ’02. Molecular mechanics calculations were carried out using MMFF force field.
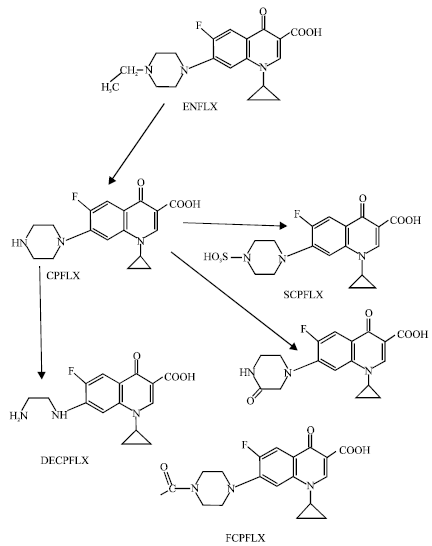 | |
| Fig. 1: | Metabolic pathways for ENFLX (Based on Andon et al., 2001 and Wang et al., 2005) |
Semi-empirical calculations were carried out using the routine PM3. DFT calculations were carried using the program Spartan ’02 at B3LYP/6-31G* level. In optimization calculations, a RMS gradient of 0.001 was set as the terminating condition. For the optimised structures, single point calculations were carried to give heat of formation, enthalpy, entropy, free energy, dipole moment, solvation energy, energies for HOMO and LUMO. The order of calculations: molecular mechanics followed by semi-empirical followed by DFT ensured that the structure was not embedded in a local minimum. To further check whether the global minimum was reached, some calculations were carried out with improvable structures. It was found that when the stated order was followed, structure corresponding to the global minimum or close to that could ultimately be reached in all cases. Although RMS gradient of 0.001 may not be sufficiently low for vibrational analysis, it is believed to be sufficient for calculations associated with electronic energy levels.
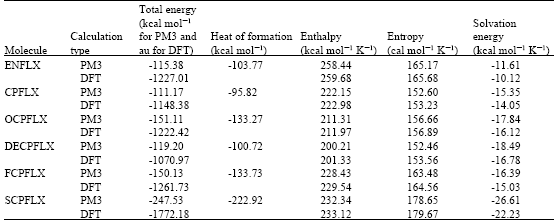
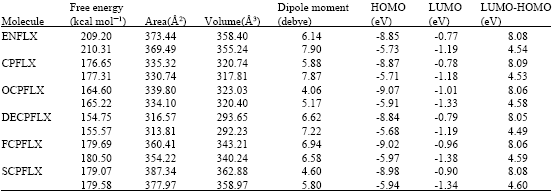
Table 1 gives the total energy, heat of formation as per PM3 calculation, enthalpy, entropy, free energy, surface area, volume, dipole moment, energies of HOMO and LUMO as per both PM3 and DFT calculations for ENFLX and its metabolites CPFLX, OCPFLX, DECPFLX, FCPFLX and SCPFLX. Fig. 2-7 give the regions of negative electrostatic potential (greyish-white envelopes) in (a) and surface charges (where red indicates negative, blue indicates positive and green indicates neutral) in (b) as applied to the optimised structures of ENFLX and its metabolites CPFLX, OCPFLX, DECPFLX, FCPFLX and SCPFLX.
| Table 1: | Calculated thermodynamic and other parameters of ENFLX and its metabolites |
 | |
 | |
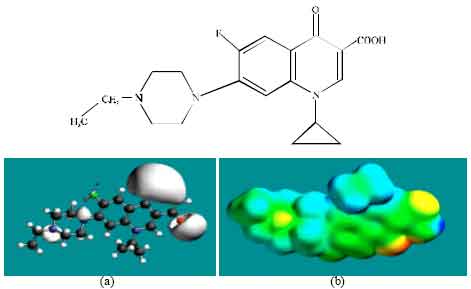 | |
| Fig. 2: | Structure of ENFLX giving in: (a) the electrostatic potential (greyish envelope denotes negative electrostatic potential), (b) surface electric charges (where red indicates negative, blue indicates positive and green indicates neutral) |
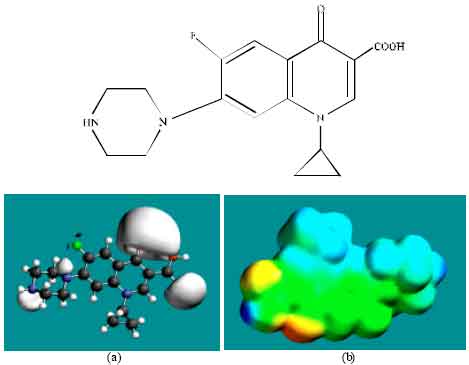 | |
| Fig. 3: | Structure of CPFLX giving in: (a) the electrostatic potential (greyish envelope denotes negative electrostatic potential) and in (b) surface electric charges (where red indicates negative, blue indicates positive and green indicates neutral) |
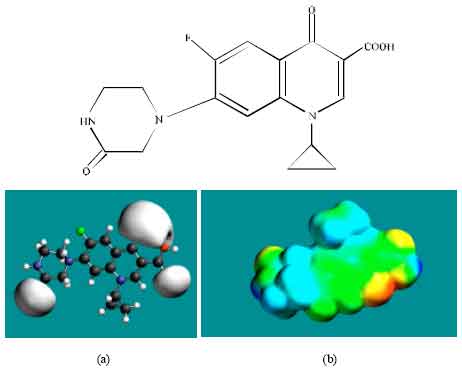 | |
| Fig. 4: | Structure of OCPFLX giving in: (a) the electrostatic potential (greyish envelope denotes negative electrostatic potential) and in (b) surface electric charges (where red indicates negative, blue indicates positive and green indicates neutral) |
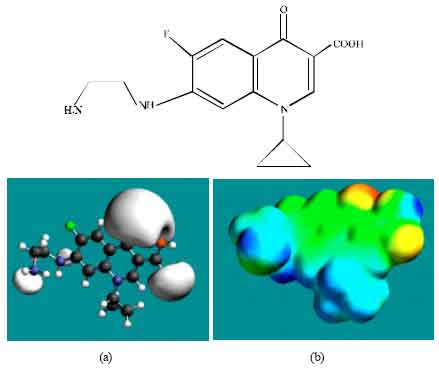 | |
| Fig. 5: | Structure of DECPFLX giving in: (a) the electrostatic potential (greyish envelope denotes negative electrostatic potential) and in (b) surface electric charges (where red indicates negative, blue indicates positive and green indicates neutral) |
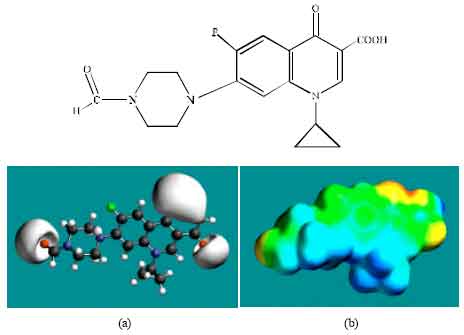 | |
| Fig. 6: | Structure of FCPFLX giving in: (a) the electrostatic potential (greyish envelope denotes negative electrostatic potential) and in (b) surface electric charges (where red indicates negative, blue indicates positive and green indicates neutral) |
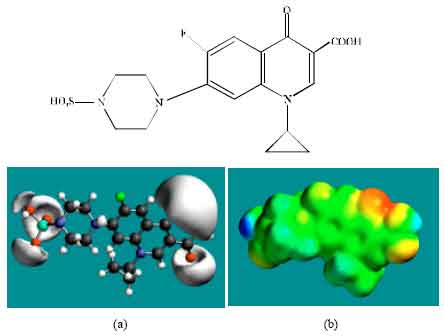 | |
| Fig. 7: | Structure of SCPFLX giving in: (a) the electrostatic potential (greyish envelope denotes negative electrostatic potential) and in (b) surface electric charges (where red indicates negative, blue indicates positive and green indicates neutral) |
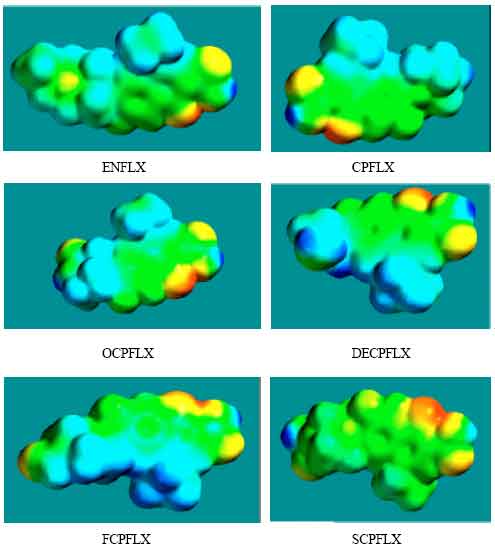 | |
| Fig. 8: | Density of electrostatic potential on the surfaces of ENFLX and its metabolites CPFLX, OCPFLX, DECPFLX, FCPFLX and SCPFLX |
The calculated solvation energies of ENFLX and its metabolites CPFLX, OCPFLX, DECPFLX, FCPFLX and SCPFLX from PM3 calculations in kcal mol-1 are respectively -11.61, -15.35, -17.84, -18.49, -16.39 and -26.61 and their dipole moments from DFT calculations are 7.90, 7.87, 5.17, 7.22, 6.58 and 5.80, respectively. The results show that the metabolites of ENFLX would be more soluble in water than the parent drug. The terminal metabolite SCPFLX would have the highest solubility in water and therefore the highest clearance rate via urine (Fig. 8).
ENFLX and all its metabolites have moderately large LUMO-HOMO energy differences (4.5 to 4.6 eV from DFT calculations) indicating that neither ENFLX nor any of its metabolites would be extremely inert or labile kinetically so that the toxicity due to ENFLX cannot be attributed solely to the parent drug or any particular metabolite.
In the case of ENFLX, CPFLX and DECPFLX, the electrostatic potential is found to be more negative around the carboxyl and carbonyl oxygen atoms and the two nitrogen atoms of the piperazine ring, indicating that the positions may be subject to electrophilic attack.
In the case of OCPFLX also, the electrostatic potential is found to be more negative around the carboxyl and carbonyl oxygen atoms, once again indicating that the positions may be subject to electrophilic attack. In the case of SCPFLX, the electrostatic potential is found to be more negative around the sulfate oxygen atoms, carboxyl and carbonyl oxygen atoms and in the case of FCPFLX, the electrostatic potential is found to be more negative around aldehydic, carboxyl and carbonyl oxygen atoms, indicating once again that the positions may be subject to electrophilic attacks.
In the case of ENFLX, CPFLX, OCPFLX and FCPFLX, HOMOs with high electron density (not shown) are found close to most of the non-hydrogen atoms of the two fused rings, the nitrogen atom of the piperazine ring that connects to the six-membered ring to which flurine is attached and the two adjacent carbon atoms of the piperazine ring whereas the LUMOs (not shown) are also found close to the all the non-hydrogen atoms of the two fused rings. In the case of DECPFLX, HOMOs with high electron density (not shown) are found to be centred on all the non-hydrogen atoms of the ring that is bonded to diaminoethyl chain and some other non-hydrogen atoms of the two terminal rings whereas the LUMOs (not shown) are found to be centred on most of the non-hydrogen atoms. The convergence or close proximity of HOMOs with high electron density with regions of negative electrostatic potential at some positions give further support to the idea that the positions may be subject to electrophilic attacks.
When the densities of electrostatic potential on the molecular surface are considered (Fig. 2d to 7d), it is found that the surfaces of FCPFLX and DECPFLX predominate more in electron-deficient regions, indicating they would be more likely subject to nucleophilic attack than ENFLX and other metabolites. However, as stated earlier, neither ENFLX nor any of its metabolites is highly reactive so that none would react readily with the key cellular antioxidant glutathione (or other biomolecules) so that depletion of glutathione cannot be a possible mechanism of toxicity due to ENFLX.
Molecular modelling analyses based on semi-empirical and DFT calculations show that ENFLX and all its metabolites have moderately large LUMO-HOMO energy differences so that none would be highly labile or inert kinetically. ENFLX and its metabolites are expected to be moderately soluble in water, except SCPFLX which would have a greater solubility. Low kinetic lability of ENFLX and all its metabolites suggests that the toxicity due to ENFLX cannot be attributed solely to the parent drug or any particular metabolite.
Fazlul Huq is grateful to the School of Biomedical Sciences, The University of Sydney for the time release from teaching.
Abbreviations
| ENFLX | : | Enrofloxacin, 1-cyclopropyl-7-(4-ethyl-1-piperazinyl)-6-fluoro-1,4-dihydro-4-oxo-3-quinoline carboxylic acid |
| CPFLX | : | Ciprofloxacin, 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinoline carboxylic acid |
| DECPFLX | : | Desethyleneciprofloxacin |
| OCPFLX | : | Oxociprofloxacin |
| SCPFLX | : | Sulfociprofloxacin |
| FCPFLX | : | Formylciprofloxacin |
| LUMO | : | Lowest energy unoccupied molecular orbital |
| HOMO | : | Highest energy occupied molecular orbital |