Research Article
Molecular Modelling Analysis of the Metabolism of Caffeine
School of Biomedical Sciences, Faculty of Health Sciences, C42, The University of Sydney, P.O. Box. 170, Lidcombe, NSW 1825, Australia
Caffeine (1,3,7-trimethylxanthine) is a widely consumed alkaloid that is present in beverages such as coffee, tea, coca products and cola drinks (Chung and Kang et al., 2000). Even decaffeinated tea and coffee still contain some amounts of caffeine. Caffeine produces increased alertness, decreased sleep and sleepiness, insomnia and increased ability to work out mathematical and cognitive problems (Smith and Reynard, 1992). At low doses it produces an increased sense of well-being and mental capacity. With larger doses the irritability may develop into what is commonly known as ‘coffee nerves’ characterized by physiological tremor, hyperactive reflexes and a modest bronchodilating effect.
Caffeine belongs to the group of biochemicals known as purines and is therefore closely related to adenine and uric acid (Galbraith and Bullock et al., 2001). Caffeine is a CNS stimulator and acts as antagonist on adenosine receptors throughout the body. Whereas adenosine generally causes lethargy, lowers heart rate and blood pressure and reduces gastrointestinal functions, caffeine causes wakefulness, high blood pressure and increased heart rate and increased gastric secretions.
Caffeine is an addictive drug. Even people who consume moderate amounts of caffeine are very much dependent on it (Galbraith et al., 2001). Acute caffeine poisoning gives early symptoms of anorexia, tremor and restlessness followed by nausea, vomiting, tachycardia and confusion. Serious intoxication may cause delirium, seizures, hypokalemia and hyperglycemia.
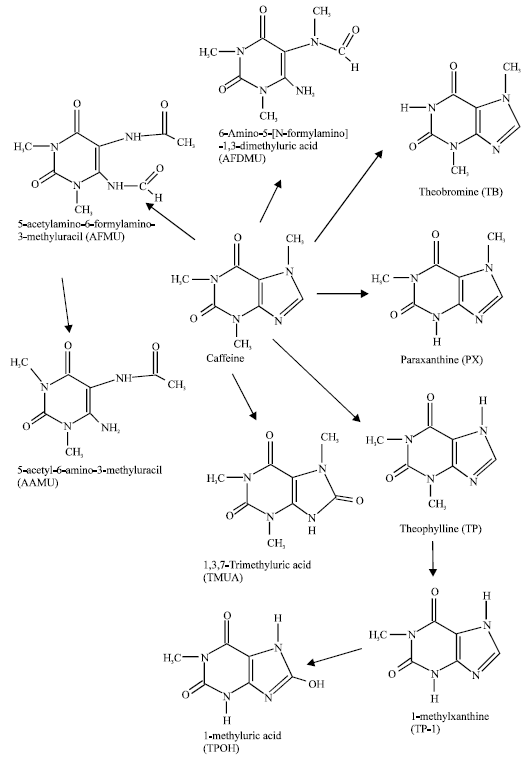 | |
| Fig. 1: | Metabolic pathways for caffeine (Tassaneeyakul et al., 1992; Nyeki et al., 2001) |
There is an increasing interest in caffeine metabolism because of the possibility of using the compound as a model substrate in studies on the activity of some of the isoenzymes of cytochrome P450 (notably CYP1A2) involved in the metabolic activation of xenobiotics (Renner et al., 1984). CYP1A2 is also known to be involved in the metabolism of a number of clinically useful drugs such as tacrine (used in the treatment of Alzheimer’s disease) and brofaromine (a selective inhibitor of monoamine oxidase A used in aged patients with mental disease). It is known that the long-term use of the drugs produce hepatotoxicity in elderly patients. Furthermore, a number of drugs may interact with methylxanthines such as caffeine that may have toxicological relevance to general population (Valero et al. 1990).
Caffeine undergoes extensive oxidative metabolism in humans and other mammalian species, initially by N-demethylation (which takes place almost exclusively in the liver) to produce theobromine (TB), paraxanthine (PX) and theophylline (TP), 1,3,7-trimethyluric acid (TMUA), 5-acetylamino-6-formylamino-3-methyluracil (AFMU), 6-amino-5-[N-formylamino]-1,3-dimethyluric acid (AFDMU) and subsequently to 1-methylxanthine (TP-1) and 1-methyluric acid (TPOH). All of these primary and secondary metabolites of caffeine are excreted in urine. N3 demethylation of caffeine reflects the activity of cytochrome P4501A2 (CYP1A2) enzyme which is responsible for the activation of numerous promutagens and procarcinogens such as aromatic amines and heterocyclic amines (Tassaneeyakul et al., 1992; Nyeki et al., 2001). Caffeine metabolite ratios such as PX:caffeine ratio have been widely used as a measure of CYP1A2 activity in humans (Casley et al., 1999).
Caffeine metabolism is well-known to be accelerated by cigarette smoking. When caffeine metabolisms of smokers and non-smokers were compared it was found that smokers had higher N1 and N3 demethylation than non-smokers (Carrillo and Benitez, 1994). Animal studies data support the idea that cigarette induces CYP2E1 activity in humans (Benowitz et al., 2003).
Figure 1 summarizes the major metabolic pathway of caffeine in humans.
In this study, molecular modelling analyses have been carried out using the programs HyperChem 7.0 (Hyper Chem, 2002) and Spartan ’02 (Spartan, 2002) to investigate the relative stability of caffeine and its metabolites.
Computation Methods
The geometries of caffeine, theobromine (TB), paraxanthine (PX) and theophylline (TP), 1,3,7-trimethyluric acid (TMUA), 5-acetylamino-6-formylamino-3-methyluracil (AFMU), 6-amino-5-[N-formylamino]-1,3-dimethyluric acid (AFDMU), 1-methylxanthine (TP-1) and 1-methyluric acid (TPOH) have been optimised based on molecular mechanics, semi-empirical and DFT calculations, using the molecular modelling programs Spartan ’02 and HyperChem 7.0. Molecular mechanics calculations were carried out using MM+ force field. Semi-empirical calculations were carried out using the routine PM3. DFT calculations were carried using the program Spartan ’02 at B3LYP/6-31G* level. For the optimised structures, single point calculations were carried to give heat of formation, enthalpy, entropy, free energy, dipole moment and solvation energy, HOMO and LUMO. The order of calculations: molecular mechanics followed by semi-empirical followed by DFT minimized the chances of the structures being trapped in local minima rather reaching global minima. To further check whether the global minimum was reached, some calculations were carried out with improvable structures. It was found that when the stated order was followed, structures corresponding to global minimum or close to that was reached in most cases. Although RMS gradient of 0.001 may not be sufficiently small for vibrational analysis, it is believed to be sufficiently low for calculations associated with electronic energy levels. This study was carried out in the School of Biomedical Sciences, The University of Sydney during the period July 2005 to February 2006.
Table 1 gives the total energy, heat of formation as per PM3 calculation, enthalpy, entropy, free energy, dipole moment, energies of HOMO and LUMO as per both PM3 and DFT calculations for caffeine, TB, PX, TP, TMUA, AFMU, AFDMU, TP-1 and TPOH. Figures 2-11 give the optimised structures of caffeine, TB, PX, TP, TP-1, TPOH, AFMU, AAMU, AFDMU and TMUA as per PM3 calculations using the program HyperChem 7.0.
| Table 1: | Calculated thermodynamic and other parameters for caffeine and its metabolites (DM stands for dipole moment) |
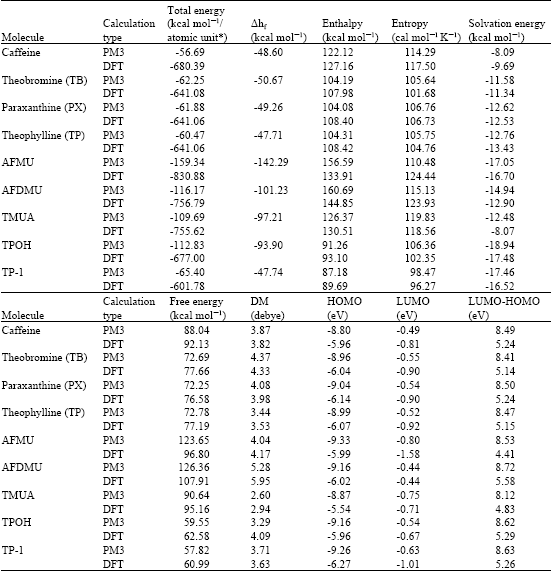 | |
| * in atomic units from DFT calculations | |
The structures also give 2D contours of total electrostatic potential. It can be seen that caffeine and all its metabolites have large negative solvation energy values indicating that the compounds would be highly soluble in water and therefore can be easily extracted in the form of urine. This means that caffeine and its metabolites will not accumulate in the body. AFMU has the lowest LUMO-HOMO energy difference (4.41 eV as per DFT calculations), indicating that the metabolite would be most labile kinetically. However, AFMU has the largest negative value for the heat of formation suggesting that the metabolite may be stable thermodynamically. It should be noted that at neutral to basic pH, AFMU spontaneously loses a formyl group to decompose into AAMU (Nyeki et al., 2001). The metabolite having the next higher LUMO-HUMO difference is TMUA. However, it has a much larger negative value of heat of formation than its parent compound caffeine, indicating that the metabolite may be more stable thermodynamically than caffeine.
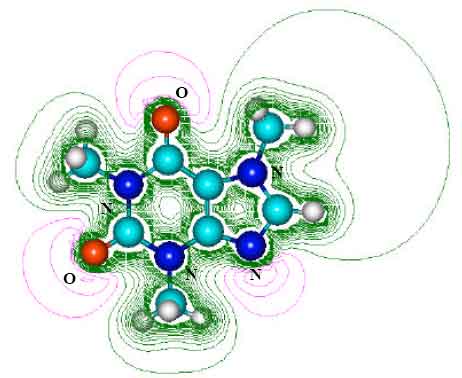 | |
| Fig. 2: | Structure of caffeine giving 2D contours of total electrostatic potential showing concentration of negative charges on oxygens and nitrogens |
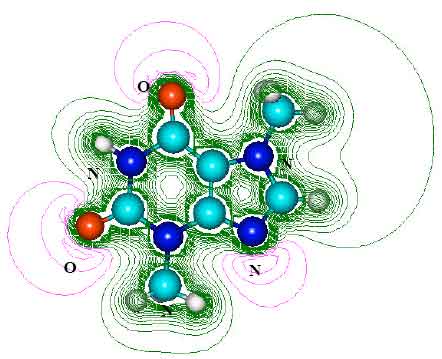 | |
| Fig. 3: | Structure of theobromine (TB) giving 2D contours of total electrostatic potential showing concentration of negative charges on oxygens and nitrogens |
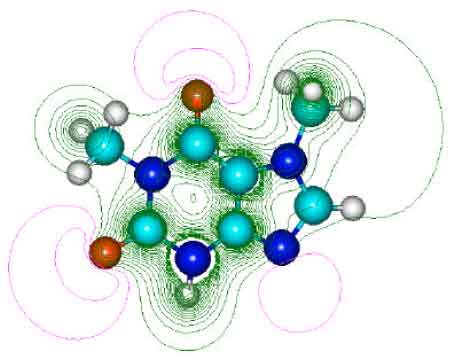 | |
| Fig. 4: | Structure of paraxanthine (PX) giving 2D contours of total electrostatic potential showing concentration of negative charges on oxygens and nitrogens |
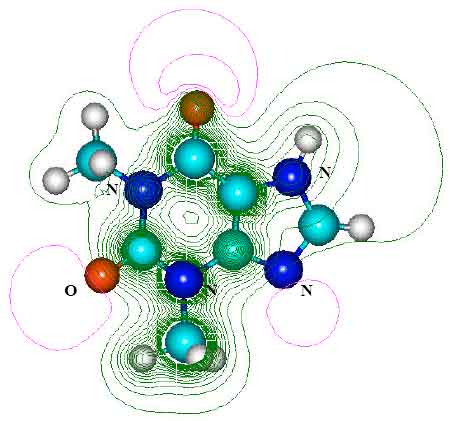 | |
| Fig. 5: | Structure of theophylline (TP) giving 2D contours of total electrostatic potential showing concentration of negative charges on oxygens and nitrogens |
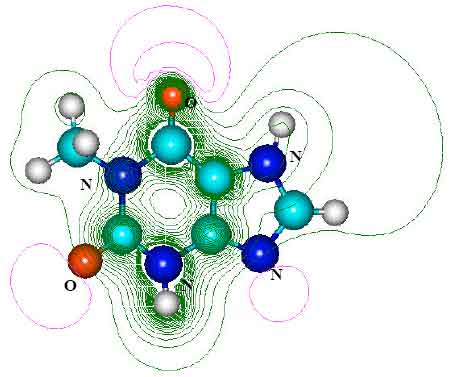 | |
| Fig. 6: | Structure of 1 - methylxanthine (TP-1) giving 2D contours of total electrostatic potential showing concentration of negative charges on oxygens and nitrogens |
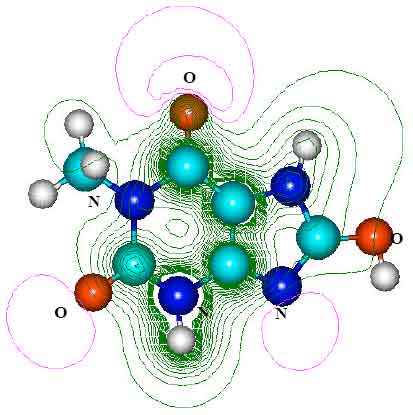 | |
| Fig. 7: | Structure of :1-methyluric acid (TPOH) giving 2D contours of total electrostatic potential showing concentration of negative charges on oxygens and nitrogens |
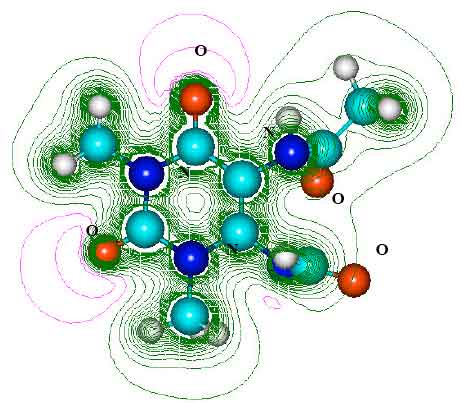 | |
| Fig. 8: | Structure of 5 - acetylamino-6-formylamino-3-methyluracil (AFMU) giving 2D contours of total electrostatic potential showing the concentration of negative charge around oxygens and nitrogens |
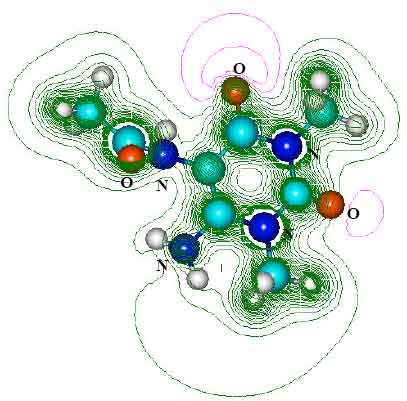 | |
| Fig. 9: | Structure of AAMU giving 2D contours of total electrostatic potential showing the concentration of negative charges around oxygens and nitrogens |
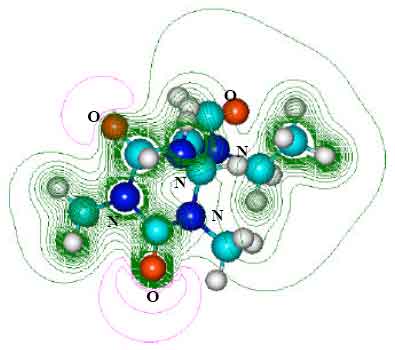 | |
| Fig. 10: | Structure of 6-amino-5-[N-formylamino]-1,3-dimethyluric acid (AFDMU) giving 2D contours of total electrostatic potential showing the concentration of negative charges around oxygens and nitrogens |
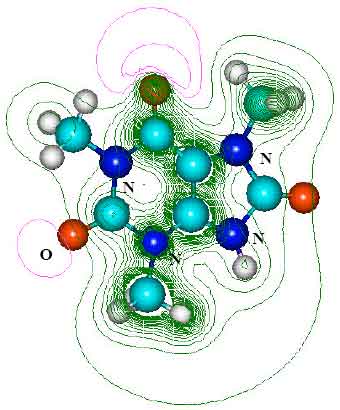 | |
| Fig. 11: | Structure of 1, 3, 7-trimethyluric acid (TUMA) giving 2D contours of total electrostatic potential showing the concentration of negative charges around oxygens and nitrogens |
Caffeine and the rest of its metabolites have moderately low values of LUMO-HOMO energy differences. Relatively low values of LUMO-HOMO energy differences for the metabolites of caffeine suggest that they would be kinetically labile so that they would be prone to further metabolism. However, as stated earlier, the high solubility of the compounds in water allow them to be easily excreted from the body via the urinary route.
Although both AFMU and AFDMU have an aldehydic functional group, the LUMO-HOMO energy difference is found to be much smaller for AFMU than AFDMU (4.41 eV for the former and 5.58 eV for the latter). As stated earlier, AFMU spontaneously loses the formyl group to form AAMU.
As stated earlier, cigarette smoking increases CYP1A2 and CYP2E1 activity so that caffeine metabolism is accelerated. Conversely if caffeine itself induces CYP1A2 and CYP2E1 activity, bioactivation of tobacco smoke carcinogens such as 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone can be accelerated from the presence of caffeine. If so, there could be added risk from smoking due to the ingestion of caffeine. CYP2E1 also metabolizes ethanol and there is no evidence that CYP2E1 activity contributes to the pathogenesis of liver disease associated with alcohol intake putatively generating free radicals and promoting lipid peroxidation (Lieber, 1999).
The contours of total electrostatic potential show the concentration of negative charges around oxygens and nitrogens, indicating that the positions may be subject to electrophilic attack.
Molecular modelling analyses show that caffeine and all its metabolites have large solvation energy values so that the compounds can be easily excreted from the body in the form of urine. Neither caffeine nor its metabolites has very small LUMO-HOMO energy difference. The high kinetic stability and high clearance rate for caffeine and all its metabolites may mean that none will be highly toxic. The metabolite AFMU has the smallest LUMO-HOMO energy difference (4.41 eV from DFT calculations) indicating that it will be somewhat more labile kinetically. It is known that AFMU spontaneously loses the formyl group to form AAMU.