Research Article
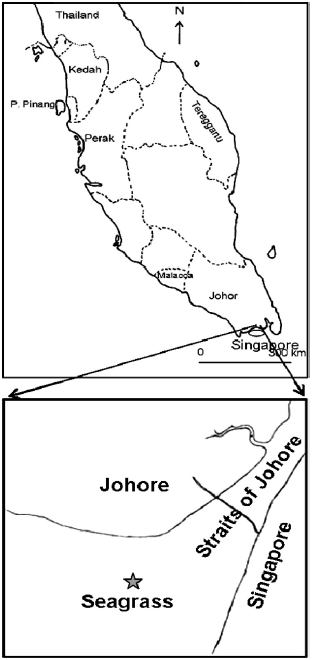
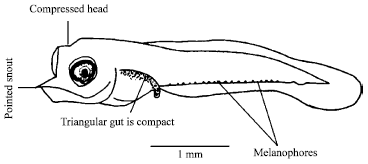
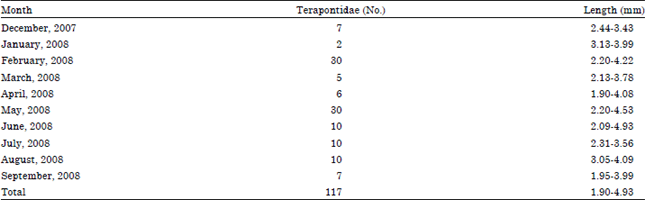
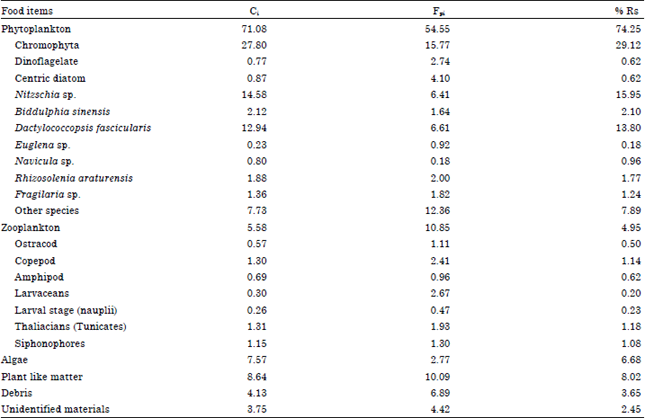
Diet Composition in Larval Fishes of the Family Terapontidae (Actinopterygii: Perciformes) in the Seagrass-bed of Johor Strait, Malaysia
Department of Aquaculture, Faculty of Agriculture, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia
R. Ara
Department of Aquaculture, Faculty of Agriculture, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia
S.M.N. Amin
Department of Aquaculture, Faculty of Agriculture, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia
A.G. Mazlan
School of Environmental and Natural Resource Sciences, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, 43600 Bangi, Selangor, Malaysia