Review Article
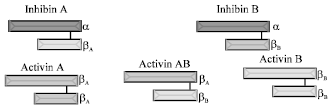
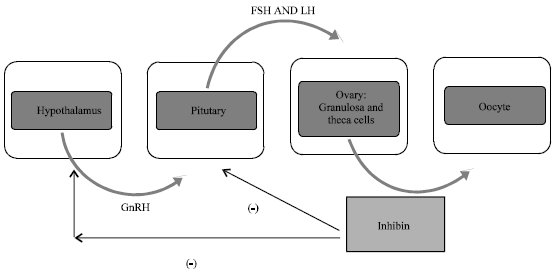
Inhibin: A Role for Fecundity Augmentation in Farm Animals
Basic and Supporting Discipline Unit, National Research Centre on Equines, Sirsa Road, Hisar-125001, Haryana, India
Varij Nayan
BPR Division, Central Institute for Research on Buffaloes, Sirsa Road, Hisar-125001, Haryana, India
Parvati
Basic and Supporting Discipline Unit, National Research Centre on Equines, Sirsa Road, Hisar-125001, Haryana, India
Mamta
Basic and Supporting Discipline Unit, National Research Centre on Equines, Sirsa Road, Hisar-125001, Haryana, India
A.K. Gupta
Basic and Supporting Discipline Unit, National Research Centre on Equines, Sirsa Road, Hisar-125001, Haryana, India