Research Article
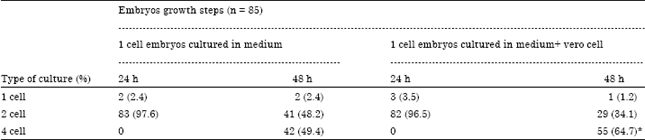
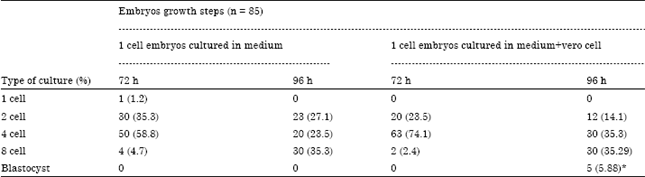
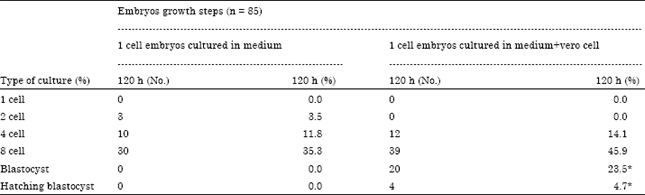
Comparison of Mouse Embryo Cleavage Rate from One Cell Stage to Blastocyst Stage in Co-culture with Vero Cells and Without Co-culture
Physiology Research Center, Ahvaz Joundishapour University of Medical Science, Ahvaz, Iran
M. Hashemitabar
Department of Anatomical Sciences, Faculty of Medicine, Ahvaz Joundishapour University of Medical Science, Ahvaz, Iran
M. Abdollahi
Physiology Research Center, Ahvaz Joundishapour University of Medical Science, Ahvaz, Iran
S.H. Razie
Physiology Research Center, Ahvaz Joundishapour University of Medical Science, Ahvaz, Iran