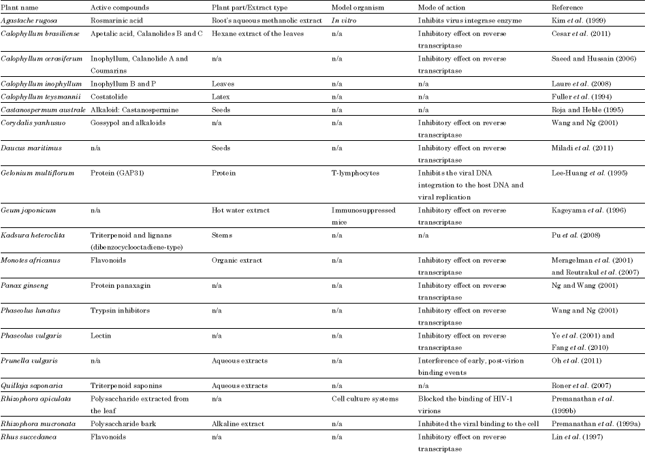
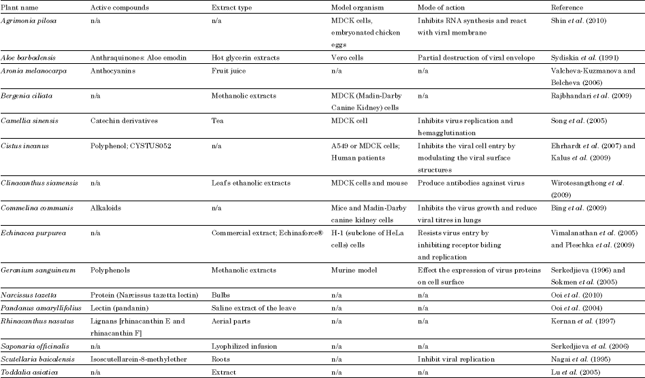
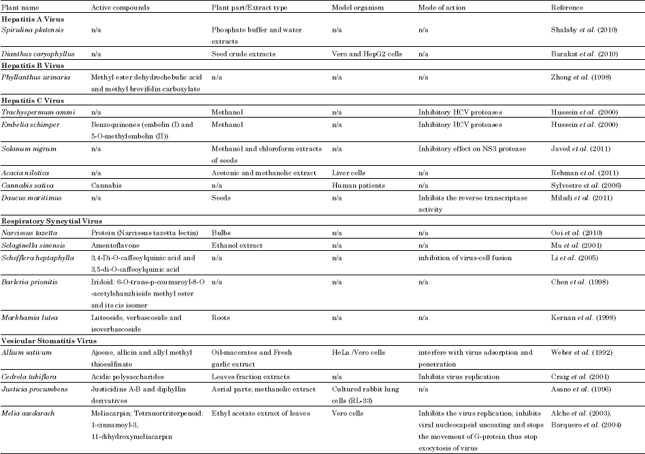
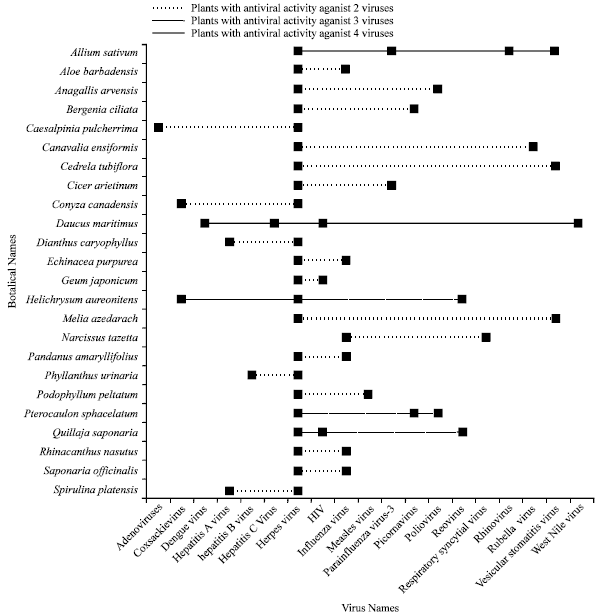
Review Article
Plant as a Source of Natural Antiviral Agents
Asian Network for Scientific Information, Faisalabad, Pakistan
Fiaz Rasul
Faisalabad Institute of Research, Science and Technology, University PARK, Near Abbas Pur, Faisalabad, Pakistan
Asia Karim
Asian Network for Scientific Information, Faisalabad, Pakistan
Uzma Kanwal
Asian Network for Scientific Information, Faisalabad, Pakistan
Idress Hamad Attitalla
Department of Botany, Faculty of Science, Omar Al-Mukhtar University, El-Bayda, Libya