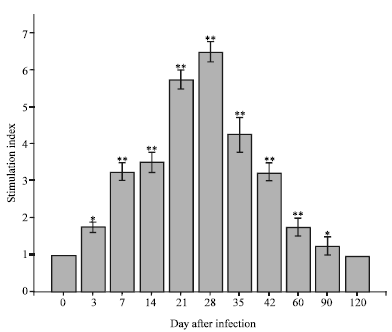
Research Article
Cellular and Humoral Immune Responses and Antigen Recognition in Sprague-Dawley Rats Experimentally Infected with Brucella abortus Biotype 1
Korean Zoonoses Research Institute, Chonbuk National University, Jeonju 561-756, Republic of Korea
M.A. Islam
Korean Zoonoses Research Institute, Chonbuk National University, Jeonju 561-756, Republic of Korea
B.K. Baek
Korean Zoonoses Research Institute, Chonbuk National University, Jeonju 561-756, Republic of Korea
S.I. Lee
Division of Model Animal, Institute of Biomedical Science, Kansai Medical University, Fumizono 10-15, Moriguchi, Osaka, 570-8506, Japan